Increased CO₂ in the ocean—what's at risk?
Increased carbon dioxide in the atmosphere also ends up in the ocean. As the ocean absorbs CO₂, the pH drops and the water becomes more acidic.
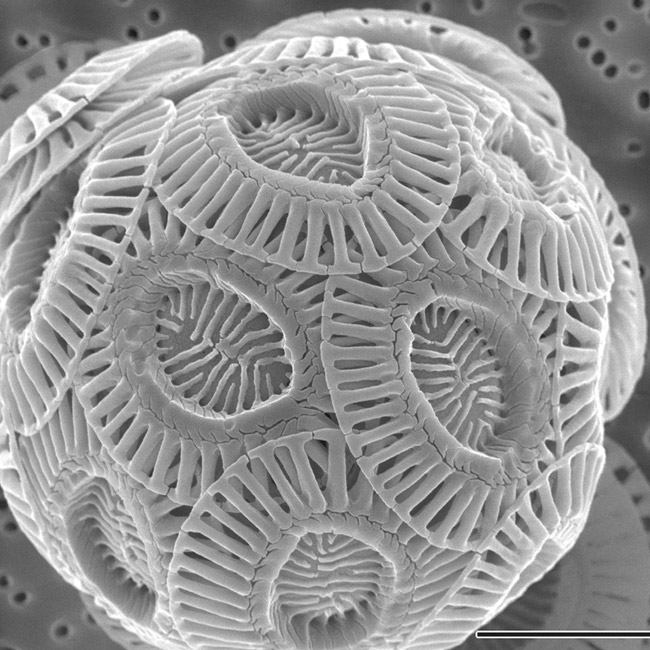
One of the life forms affected by ocean acidification is a type of phytoplankton called coccolithophores. These are one of the most abundant single-celled algae in the ocean. They are found in the upper sunlit layers of the sea and play a vital ecological role. Coccolithophores produce a large proportion of the planet's oxygen, sequester huge quantities of carbon and provide the primary food source for many of the ocean's animals.
Coccolithophores use calcium carbonate in the form of calcite to form tiny plates, or scales, on their exterior. Oceans with a lower pH that can dissolve calcium carbonate could therefore have a harmful effect on the abundance of coccolithophores and, consequently, on the health of the oceans and the planet.
Another type of plankton important to the marine food web are pteropods. Also known as ‘sea butterflies’, these are tiny swimming marine snails with shells made of aragonite, a type of calcium carbonate. A study has found the shells of pteropods in the Southern Ocean near Antarctica are already showing signs of dissolving in waters with an aragonite saturation state of around 1.
Ocean acidification could also damage corals, such as those in the Great Barrier Reef. Corals mainly use aragonite to build their skeletons. Ocean acidification could limit the formation of new corals, weaken existing corals, and exacerbate the problems associated with coral bleaching and storm damage.

Some shellfish will struggle to make their shells, with the larval stages particularly vulnerable. Along with difficulties in shell growth, changes in pH and carbonate chemistry also make it more difficult for some animals to regulate their cell chemistry. They may end up spending more energy on growing their shells, detracting from their ability to grow, reproduce or cope with other stresses.
However, as with all natural systems, things are rarely black and white. A team of researchers from Woods Hole Oceanographic Institution have found that not all calcifying animals react in the same way to lowered pH conditions.
Although some animals and plants may not be as adversely affected as others, the impacts on marine biodiversity have the potential to be severe. Changes that threaten coccoliths and plankton will put pressure on the broader marine food chain. In addition, around 25 per cent of ocean animals depend in some way on coral reef systems so if these decline the ramifications will be far-reaching.





