Earth's sunscreen, the ozone layer
Expert reviewers
Professor Neville Fletcher AM FAA FTSE
Emeritus Professor, University of New England
Visiting Fellow, Australian National University and adjunct professor at The University of New South Wales
Essentials
- Ozone is a form of oxygen in our upper atmosphere that protects the planet
- Almost all living things would suffer radiation burns if we didn't have the ozone layer to protect us
- Human activity has led to ozone depletion
- Ozone-depleting substances are generally very long-lived, as they take years to drift upwards and interact with the upper atmosphere
- Due to international banning and controlling of these substances, it’s believed that the biggest ozone holes will recover by around 2040
Up in the stratosphere, small amounts of ozone are constantly being made by the action of sunlight on oxygen. At the same time, ozone is being broken down by natural processes. The total amount of ozone usually stays constant because its formation and destruction occur at about the same rate.
Human activity has recently changed that natural balance. Certain manufactured substances (such as chlorofluorocarbons and hydrochlorofluorocarbons) can destroy stratospheric ozone much faster than it is formed.
Ozone is a natural sunblock
Go outside on a fine day and feel the sun warm your face. What happens when a cloud passes over? You’ll notice that the cloud takes away some of the heat and light coming from the sun. In much the same way that a cloud blocks the heat on a hot day, the ozone layer in the stratosphere blocks out the sun’s deadly ultraviolet rays. It acts as our planet’s natural sunblock.
The sun doesn’t just produce heat and light. It throws out all sorts of other types of electromagnetic radiation, including ultraviolet radiation. Because ultraviolet radiation can damage DNA it is potentially harmful to most living things, including plants.
Unfortunately our bodies can't detect ultraviolet radiation directly. We can be unaware of the harm it is doing until it is too late—for example, at the end of a day in the sun without adequate protection.
When there is less ozone in the stratosphere, more ultraviolet radiation hits us.
Even a 1 per cent reduction in the amount of ozone in the upper atmosphere causes a measurable increase in the ultraviolet radiation that reaches the Earth's surface. If there was no ozone at all, the amount of ultraviolet radiation reaching us would be catastrophically high. All living things would suffer radiation burns, unless they were underground, in protective suits, or in the sea.
What exactly is ozone?
Ozone is a form of oxygen. Each ozone molecule is made of three oxygen atoms, so its chemical formula is O3. But unlike oxygen, ozone is a poisonous gas, and an increase in its concentration at ground level is not something that we want. But in the stratosphere, where ozone exists naturally, it blocks out the sun's ultraviolet rays and is a life-saver.
Ozone-depleting substances usually contain chlorine or bromine.
The synthetic chemicals called chlorofluorocarbons (CFCs) are now well-known as environmental ‘baddies’, even though they are useful and completely non-toxic substances. They get their bad name because they are ozone-eaters (properly called ozone-depleting substances). CFCs are not the only ozone-depleting substances, but they are the most abundant. Some ozone-depleting substances are naturally occurring compounds.
Ozone-depleting substances are generally very long-lived, and it takes several years for them to to drift up into the stratosphere. When they arrive, they are broken apart by exposure to ultraviolet radiation and that releases the chlorine atoms. These are the real ozone-killers. The chlorine atoms react with ozone, to form oxygen and chlorine monoxide.

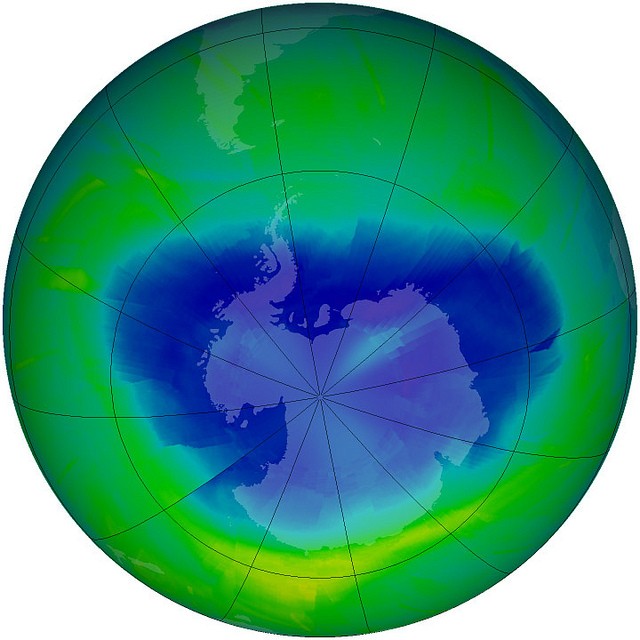
Ozone depletion
The ozone-destroying reactions take place most rapidly only under certain conditions in the stratosphere. These conditions—extreme cold, darkness and isolation, followed by exposure to light—occur over the polar regions after the long polar winter has finished and the first spring sun appears.
Antarctica is the worst affected area, probably because the air above it is most isolated from the rest of the atmosphere. Scientists often refer to the part of the atmosphere where ozone is most depleted as the ‘ozone hole’, but it is not really a hole—just a vast region of the upper atmosphere where there is less ozone than elsewhere. At its most extreme, the 'ozone hole' contains 60 per cent less ozone than normal.
Ozone-poor air can spread out from the polar regions and move above other areas. In addition, direct ozone loss elsewhere is slowly increasing, although it is not occurring at the same rate as over the poles.
Scientists around the world regularly monitor ozone-depleting substances and the amount of ozone in the stratosphere. In Australia, the Australian Bureau of Meteorology and the CSIRO Division of Atmospheric Research jointly manage the Cape Grim Baseline Air Pollution Station. The Cape Grim station is located in remote north-western Tasmania, in the path of strong westerly winds that carry air thousands of kilometres across the Southern Ocean. Air at Cape Grim is regularly sampled in order to monitor atmospheric composition. (Another reason to monitor ozone-depleting substances is because most are also powerful greenhouse gases.)
Reversing the depletion
Banning and controlling ozone-depleting substances
Many substances other than chlorofluorocarbons are also ozone-depleting. Examples are carbon tetrachloride (used in dry cleaning), and methyl bromide (used as an insecticide for soil fumigation). An Australian scientist (Jonathan Banks) has been internationally recognised for his work in finding a replacement for methyl bromide.
CFCs, previously used as refrigerants, foam-blowing agents and propellants in spray cans, are now banned in Australia (and many other countries). Their temporary replacements, the hydrochlorofluorocarbons, are still slightly ozone-depleting, though not to the same extent. HCFCs are also being phased out.
An international agreement to address the problem of ozone depletion was devised in 1987. Called the Montreal Protocol, this agreement limits the production and use of ozone-depleting substances. By 2009, the Montreal Protocol had been agreed to by all United Nation (UN) member states, making it the most widely ratified treaty in UN history. It was agreed that all CFC production world wide would be stopped by 1 January 2010. By September 2011, nearly 100 ozone depleting substances (amounting to 97 per cent of the substances controlled by the Montreal Protocol) had been phased out.
A slowing down in the rate of ozone loss has been measured, and the concentration of CFCs in the atmosphere is slowly levelling off. But because of the long-lived nature of the ozone-depleting substances, they will hang around and continue doing their nasty work for a long time after their actual production has stopped. It is estimated that it will take decades to reverse the problem, though scientists are hopeful that the biggest holes in the ozone layer will recover by around 2040.





