Fighting back: antimicrobial resistance
Expert reviewers
Professor Graeme Nimmo FRCPA, FASM
State Director of Microbiology for Pathology Queensland
Griffith University Medical School
Affiliate Professor John Turnidge
Affiliate Professor, School of Molecular and Biomedical Sciences
University of Adelaide
Essentials
- Antimicrobial resistance (AMR) threatens to make existing standard medical treatments ineffective.
- This means that currently common and treatable infections are becoming life threatening.
- The World Health Organization describes antibiotic resistance as one of the greatest threats currently facing human health.
- AMR is present in all parts of the world.
- AMR affects humans, animals and agriculture.
Imagine an ear infection that leads to hearing loss, or a kidney infection that causes permanent renal failure. Worse still, imagine taking a loved one to hospital for minor surgery, only to watch them die within days from a range of post-operative infections. This is the stark reality of antimicrobial resistance, a world in which currently common and easily treatable infections become life-threatening.
Without urgent, coordinated action, the world is heading towards a post-antibiotic era, in which common infections and minor injuries, which have been treatable for decades, can once again kill.World Health Organization (WHO)
What is antimicrobial resistance?
At its simplest, antimicrobial resistance (AMR) is when micro-organisms such as bacteria, parasites and viruses are able to survive in the presence of antibiotics, antivirals and other anti-infective agents that previously killed them and cured the infection. This resistance results in severe prolonged infections that may become life-threatening and allows the micro-organisms to survive and spread to other people.
Antibiotics vs antimicrobials
Antibiotics are effective against bacteria, just one class of micro-organism, while the term antimicrobial resistance (AMR) covers the development of resistance in all micro-organisms—including bacteria, fungi, viruses and protozoa (such as the parasite that causes malaria)—to the various agents used to treat the infections they cause.
Antibiotics and other antimicrobial agents (collectively known as antimicrobial agents) have been an important part of modern medicine since the 1930s. Their use has resulted in improved health and increased life-span, and has enabled many of the medical breakthroughs—such as transplantation and cancer therapy—we now take for granted. However, they have been used for so long and so widely around the world that many of the infectious organisms they are designed to eliminate have adapted and become resistant to them.
Superbugs
When bacteria become resistant to multiple types of antibiotics, they are often called ‘superbugs’. In the past, most of these superbugs were confined to healthcare settings such as hospitals and nursing homes, where patients already in a weakened condition were more susceptible to picking up infections.
More recently, superbug infections have started to appear in the general community due to high levels of unnecessary antibiotic use for conditions such as common colds, (which are due to viruses that don’t respond to antibiotics anyway). Therefore, even healthy people are at risk of untreatable bacterial infections. There are currently several types of ‘superbug’ in the community, including Methicillin-resistant Staphylococcus aureus (MRSA), Staphylococcus aureus (commonly known as Golden Staph), multi-resistant Escherichia coli (commonly called E.coli, causing urinary infections), the serious diarrheal infection Clostridium difficile, and Neisseria gonorrhoeae, among others.

What causes AMR?
There are two main causes for AMR: evolution, and human behaviour.
Evolution
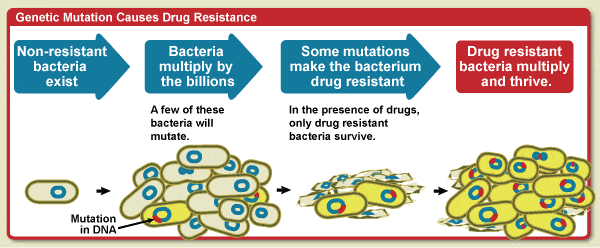
All living things evolve, but bacteria, viruses and parasites are experts at it, and do it very rapidly. Developing resistance to antimicrobials can occur naturally whenever they are used and can happen in several ways, through spontaneous mutation and via the transfer of genes.
Spontaneous mutation in the bacterium’s DNA
Mutations—spontaneous changes in a bacterium’s genetic material—occur in about one in one million to one in ten million cells. Depending on the mutation, different types of resistance can occur. For example, mutations can:
- provide the ability to close or seal up the entry ports through which antibiotics enter a bacterial cell
- modify the bacterial cell target that the antibiotic is programmed to attack
- increase the production of natural enzymes that inactivate antibiotics
- allow the bacteria to increase the production of natural pumping mechanisms that expel the antibiotic for the bacterial cell so that it fails to reach its target.
These mutations enable the bacteria to survive or resist the effects of the antibiotic. These ‘new and improved’ model bacteria stay alive and continue to replicate and multiply.
Transfer of antibiotic genes
We might think bacteria are nasty, but they actually have very good manners—at least when it comes to sharing. Antibiotic-resistant bacteria can share their genetic defences (essentially pieces of their DNA) with other bacteria via a simple mating process called ‘bacterial conjugation’. This is accomplished via direct cell-to-cell contact or by a bridge-like connection between two cells.
Resistance traits can also be passed between bacteria by the viruses that infect them. The resistance traits from one bacterium are packaged into the head portion of the virus. The virus then injects the resistance traits into any new bacteria it attacks. Bacteria also have the ability to acquire free or naked DNA (that is, DNA that is released from another cell) from their environment.
Microbiologist Professor John Turnidge says bacteria literally borrow their resistance genes from neighbouring bugs. ‘They’re the original life forms….so for thousands of millions of years they’ve had a chance to work out ways to survive and one of those is to borrow genes from other bacteria to survive.’
Resistance genes, whether acquired by spontaneous mutation or genetic exchange, enable bacteria to resist antibiotics. But why stop at one? Bacteria can and do collect multiple resistance genes over time, resulting in resistance to many different types of antibiotics and thus creating superbugs that may be impossible to treat.
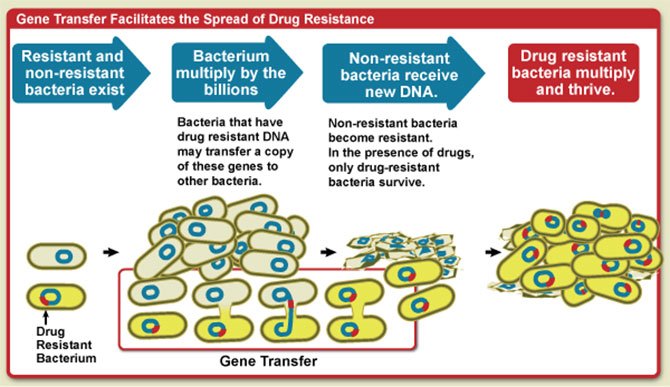
An illustrative diagram that shows how gene transfer facilitates the spread of drug resistance. Image source: NIAID / Flickr
Human behaviour
Human reliance on antibiotics
The natural processes, including how quickly and to what extent antimicrobial resistance occurs, have been determined by the second reason for AMR: human behaviour.
The increasing availability and low cost of antibiotics has resulted in their inappropriate use all around the world. This includes their over-use, misuse and underuse in human health, animal husbandry and agriculture. This inappropriate use has been the key driver of increased antibiotic and antimicrobial resistance globally. The emergence and spread is increasing at a pace that exceeds the pharmaceutical industry’s capacity to develop new antimicrobial drugs.
Australia has one of the highest antibiotic prescribing rates in the world with around 30 million prescriptions written every year, of which up to half are estimated to be unnecessary. Often, doctors will prescribe antibiotics ‘just in case’, or because they’re not sure exactly what’s wrong, or simply because patients expect them. A common misconception by patients is that antibiotics will help cure common ailments such as colds, flu, sore throats and bronchitis. However, these are all caused by viruses (as opposed to bacteria), so antibiotics will make no difference to a person's recovery time.
Dr. Phillipa Binns, clinical advisor at NPS MedicineWise, said that ‘Every time you take antibiotics you are increasing the chances that next time you use that particular antibiotic, it won't work as effectively … you're also making it more likely that you could get infections that can't be properly treated.’ That’s right, taking even one course of antibiotics can contribute to AMR.
Australia is not the only country with an antibiotic problem.
Coupled with the overuse of antibiotics, the misuse of antibiotics is also a problem. This includes not following instructions correctly, taking medications purchased online or abroad after self-diagnosis, consuming antibiotics from a previous script or that belong to someone else, and incorrect dosage or delivery method. Failing to take antibiotics correctly allows more opportunity for the bacteria in your system to become resistant.
Antibiotics in livestock
Our reliance on antibiotics goes beyond just ourselves and our own medication.
Antibiotics are also widely used in the animal industry, in the farming of meat, poultry and fish. Approximately 700 tonnes of antibiotics are imported into Australia each year, with around 550 tonnes (78 per cent) being used for ‘disease control’ in food animals or for the treatment of sick animals. AMR in animals can result in reduced animal health and production outcomes. It is also possible for AMR bacteria in animals to be transferred to humans by direct contact or through food.
Antibiotics in agriculture
In agriculture, antibiotics are also used. For example, they can be sprayed onto fruit trees to prevent and treat infection, (although this is not as common in Australia as it is in other countries). After the initial spraying, a few bacteria survive among the traces of antibiotics that remain, possibly encouraging the emergence of resistant strains of bacteria. Low concentrations of these antibiotics can also be spread further afield via wind or water, providing more opportunity for resistant bacteria to develop.
Measureable amounts of antibiotics have also been found in water and soil, having found their way there via contaminated effluent and manure from humans and animals.
In Australia, spraying crops with antifungal agents is also an issue, as the classes of antimicrobials used for this are sometimes the same as those used in the human health context.
So, humans, animals and plants are all being affected by AMR, and the consequences could be catastrophic.
 opener
opener
Antimicrobial resistance in animals can be transferred to humans by direct contact or through food. Image source: Jennikate / Flickr
Why should I be concerned about AMR?
Antimicrobial resistance has been called one of the world's most pressing public health problems.
You might think ‘that’s interesting, but it doesn’t affect me. I’m fit and healthy and I’ve rarely taken these medications’. But you’re not off the hook. AMR can affect any person, of any age or fitness level, in any country. The reason? It’s not the individual who becomes resistant to antimicrobial medication, rather, resistance is a property of the micro-organism, and almost every type of bacteria has acquired resistance and become less responsive to antibiotic treatment since the 1940s.
If you are unlucky enough to catch an antibiotic-resistant infection, you:
- will have the infection for longer
- may be more likely to have serious complications from the infection or even be killed by it
- could remain infectious for longer, and pass your infection to other people, which increases the problem.
Some experts have warned that AMR threatens to take us back to the pre-antibiotic era, but actually, it’s worse than that. This will be a post-antibiotic era, where the germs are stronger and more resilient and replacement antibiotics are rare. Imagine an infected cut that becomes life threatening, or pneumonia once again becoming a mass killer. Many of the medical advances we currently take for granted such as cancer treatment, transplantation, surgery and neonatal care―all of which rely on antibiotics to control infection―would be impossible.
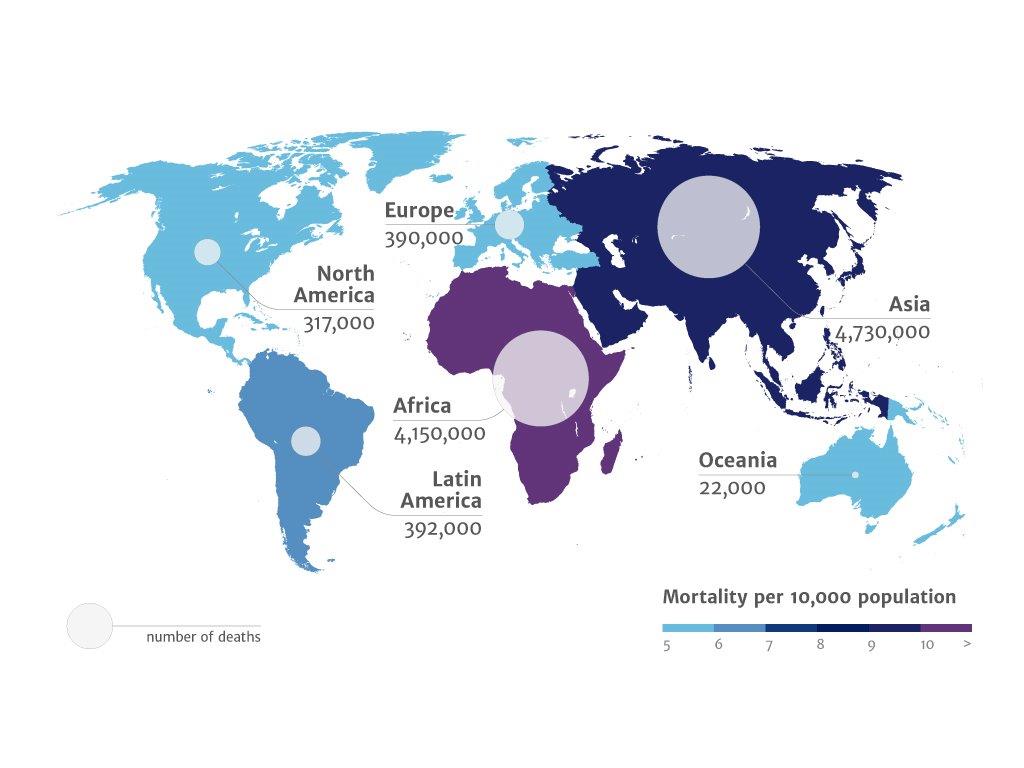
This is not a future scenario, but one that is happening right now. In 2014, both Europe and the US estimated their current deaths from AMR to be 25,000 and 23,000 respectively, though experts believe these figures to be conservative.
A report commissioned by the British government in 2013 estimated that global deaths from AMR are currently 700,000, but that that by 2050 AMR could be responsible for 10 million deaths per year and at least $100 trillion (more than the size of the current world economy) in lost output. Both these figures are conservative, because the report only looked at the impact of six antibiotic-resistant pathogens, and did not include the costs of healthcare or secondary social costs.
While AMR can affect anyone, in any country, the burden of antimicrobial resistance is not evenly distributed around the globe. Poorer countries and those with restricted access to modern health care feel the burden of AMR more fully than their more developed neighbours. For example, if the present situation continues, it is estimated that by 2050 one-quarter of all deaths in Nigeria could be caused by AMR.
If we fail to act, we are looking at an almost unthinkable scenario where antibiotics no longer work and we are cast back into the dark ages of medicineDavid Cameron, UK Prime Minister
Why not just make new antibiotics?
It sounds simple, doesn’t it? If bacteria are becoming resistant to current antibiotics, why not just develop new ones? But developing a new antibiotic doesn’t happen overnight. In fact, there have been no wholly new antibiotics in over a decade. There are several reasons for this.
Economic
It may sound callous, but from a commercial point of view, there’s really not that much money to be made from antibiotics. In 2015, only six of the world’s top 50 pharmaceutical companies are still pursuing antibiotic research. After all, why would major pharmaceutical companies invest time and money developing drugs that are only taken for a short time to achieve a cure, when they could be developing drugs to treat chronic illness (such as high blood pressure or cholesterol) that are taken for life?
In addition, any new antimicrobial will inevitably have its usefulness reduced by the development of resistance in target micro-organisms.
Scientific
Anitmicrobials are complex and need very large investments in research and development before they can be marketed. Most never make it to market and many companies state the scientific challenges of antibiotic development as disincentives to continue further research. One oft-repeated line is that the ‘low hanging fruit has been picked’, which basically translates as ‘the easy or basic antibiotics have been found, and now it’s much harder to find and develop new ones’. Indeed, drug screens for new antibiotics tend to re-discover the same lead compounds time and again. Thus, discovery and development of antibiotics has become scientifically more complex and more time consuming—and therefore more expensive.
Taking the baton from the large pharmaceutical companies, small and medium biotech firms have become the innovators in the industry, working to find new antimicrobials which they hope will then be acquired by their larger counterparts. At the end of the day however, money and profit is still the key driver in this industry.
Regulatory
Getting a new drug approved is never easy. There are multiple regulatory requirements that companies must manoeuvre and red tape that must be cleared. The difficulty of the process, coupled with the scientific and economic considerations, have resulted in many antimicrobials being placed in the ‘too hard’ basket.
To combat the lack of interest from major companies, the US Government has stepped in with public incentives, patent protection, fast-track approval processes and partnerships to try to promote interest in these ‘forgotten drugs’. In 2015 President Barack Obama dedicated US$1.2 billion from the annual budget to fight against AMR infections. It is hoped that this investment may go some way towards reviving interest in antibiotic development.
What is being done to address AMR?
The seriousness of AMR is being recognised around the world, and a global effort is now underway to try to reduce and control antibiotic resistance.
In 2012, the World Health Organization (WHO) released its report The Evolving Threat of Antimicrobial Resistance: Options for Action. This important document identified several key measures to help fight AMR, including collecting national and international data on antimicrobial use and levels of resistance; ensuring correct and appropriate use of antimicrobial drugs in both humans and animals; education and coordination programs to limit the spread of infection; and supporting research and innovation into new antimicrobial drugs through effective partnerships and programs.
With WHO leading the way, many countries around the world have now implemented national strategies and procedures to try and tackle AMR. The Australian Government, through the departments of health and agriculture and with advice from the Australian Strategic Technical Advisory Group on Antimicrobial Resistance (ASTAG), has produced a national strategy entitled Responding to the Threat of Antimicrobial Resistance. The strategy specifies seven objectives including effective antimicrobial stewardship (more appropriate use), better infection prevention, more research, and enhanced surveillance of antimicrobial resistance and antimicrobial usage.
In addition, several Australian agencies and organisations, such as NPS Medicinewise, have created large public-awareness campaigns to help educate the public about AMR and the ways that individuals can try to stop it.
What can I do to help?
While AMR is global issue, there are small things you can do that will make a difference. You can:
- get vaccinated, and keep your vaccinations up to date
- only take antimicrobial drugs when directed by a health professional and always ask if they are really necessary
- follow the directions closely and never share medications with others
- try to avoid close contact with sick people to prevent the transmission of bacterial and viral infections
- wash your hands correctly with soap—yes soap, not antibacterial handwash, which several studies have linked to increasing AMR—and warm water for at least 20 seconds
- ensure good hygiene at all times.

Conclusion
Antimicrobial resistance is present and increasing right now across the world. Antibiotics are becoming less effective and some micro-organisms are now resistant to all available antimicrobials. This poses serious risks for global public health, where common infections and minor injuries, which have been treatable for decades, can once again kill.
Many countries are now taking the threat of AMR seriously and are putting strategies in place to help combat it―including surveillance, education campaigns, policy change and funding. At an individual level, we can ensure we only take antimicrobial drugs when really necessary, follow all instructions correctly, and work to keep ourselves and our families healthy.
We can hope that AMR will be the impetus for a new wave of innovation and improvement in healthcare, not only for the type of drugs we take, but also how and why we take them.






