What’s blue carbon?
Expert reviewers
Essentials
- Increased levels of carbon dioxide in the Earth’s atmosphere are contributing to the warming of our planet.
- Plants play an essential role in the global carbon cycle by removing carbon dioxide from the atmosphere and storing it as organic carbon in biomass and sediments.
- Carbon captured by oceans, marine plants and coastal ecosystems is known as blue carbon.
- Coastal ecosystems are very efficient at sequestering carbon and can store it for thousands of years.
- Human activities have led to the destruction of a significant number of these important blue carbon sinks, with more under threat.
Our planet is getting warmer—and with warmer temperatures comes a range of consequences. Some we’re already seeing, while others are likely in the future: from increased severity and occurrence of coral bleaching events, to the movement of species towards the poles and long-term changes in rainfall patterns.
A major contributor to these higher temperatures is increased levels of carbon dioxide—CO2—in the Earth’s atmosphere. Carbon dioxide is what’s known as a greenhouse gas. By preventing some of the heat radiated from Earth’s surface from leaving the atmosphere, it traps heat, keeping our planet warm enough for us to live on—just like a greenhouse keeps plants toasty. When there’s too much carbon dioxide in the atmosphere, however, things really start heating up—like on Venus, where an excessive greenhouse effect has elevated temperatures to extreme levels.

Trees and other plants need carbon dioxide to grow and, in the process of doing so, they remove it from the atmosphere and store it as organic carbon. This is one of the reasons why forests are so important for the health of our planet. Not only do they provide habitat for many species, but they’re also what’s known as carbon sinks—they remove carbon dioxide from the atmosphere and convert it to organic carbon compounds, a portion of which is stored (sequestered) for decades, and sometimes longer, in the plant’s biomass and in the soil.
But it’s not just forests which are important when it comes to keeping carbon dioxide in our atmosphere at manageable levels. Oceans, marine plants and, especially, marine plants of coastal ecosystems also play a vital role in dealing with carbon dioxide. While coastal ecosystems take up less space than forests on land, their higher levels of carbon sequestration per area make them just as important in the global carbon budget. Not only that, but they can store carbon for very long periods of time—not just decades, or even centuries, but thousands of years. The carbon captured by these coastal ecosystems has become known as blue carbon.
Before we get to the blue stuff, though, let’s take a step back and look at carbon itself.
Carbon: what’s the big deal?
Carbon footprint, carbon neutral, carbon credits … they’re phrases that get thrown around in the media on a daily basis. But what is carbon exactly? Why is too much a problem? And why is it so important that there are ways of capturing and storing it?
WHAT IS CARBON, EXACTLY?
Carbon is a chemical element which is fundamental to all life on Earth. Its symbol is C, and it’s number 6 on the periodic table. Carbon compounds are sometimes called the building blocks of life, as all living things (you and me included) are based on carbon and carbon compounds. In fact, around 18.5 per cent of the human body is carbon. There are a huge number of carbon compounds, and they can exist in various forms—as solids (like coal), liquids (like crude oil) and gases (like carbon dioxide). The black stuff on top of burnt toast is a form of carbon (amorphous carbon), as are diamonds.
THE CARBON CYCLE
There’s actually a fixed amount of carbon on Earth. But it’s constantly changing into different forms, and is cycled back and forth between living and non-living things, and exchanged between the earth, the atmosphere and oceans. This is known as the carbon cycle.
Here’s how it works.
1. Plants take in carbon dioxide from the atmosphere and, through photosynthesis GLOSSARY photosynthesisthe process used by plants and other organisms to turn light energy into chemical energy for fuel. The basic equation for photosynthesis is: carbon dioxide plus water (in the presence of light) results in sugar plus oxygen. , release oxygen (pretty handy for us, since we need it to survive).
2. The carbon dioxide is converted into organic carbon compounds, which make up the plant’s body. These compounds are stored in the plant’s shoots and leaves, as well as below ground in its roots.
3. Animals eat the plants to get the carbohydrates which they need for energy, and other animals eat those animals. They also breathe in the oxygen released by plants, and breathe out carbon dioxide.
4. The carbon dioxide is then taken up by plants, and the cycle begins again.
5. The carbon that hasn’t been eaten decomposes when the plant or animal dies. It either gets released back into the atmosphere or can be stored in the soil.
6. As well as this biological cycling, carbon is also exchanged between the atmosphere and the oceans.
Some plants—especially squishy ones, like tomatoes, for example—decompose pretty quickly, so they don’t hang on to carbon for very long. This means that there’s a portion of carbon that runs through the whole cycle (going from CO2 in the atmosphere, to carbon in plants and animals, and back into CO2 in the atmosphere) pretty quickly, relatively speaking.
But another portion of carbon is stored for a very long time—up to thousands of years. An example is the partly decayed organic matter from those plants and animals that managed not to get eaten. When exposed to heat and pressure in the Earth’s crust over long time periods, these are converted to carbon compounds such as coal or crude oil—known as fossil fuels.
When people use fossil fuels for energy, what they’re doing is taking these carbon-based compounds from the ground and burning them, releasing carbon dioxide in the process. Black coal, for example, is a fossil fuel known as a hydrocarbon; it contains carbon and hydrogen, as well as a few other things. When burnt, the carbon and hydrogen combine with oxygen from the air, producing water vapour (steam, which is usually used to power turbines that produce electricity) and carbon dioxide, which is released into the atmosphere. So, by releasing carbon that’s been sequestered by plants a a long time ago, we’re contributing to an excess of carbon dioxide in the Earth’s atmosphere which, in turn, traps the sun’s heat, contributing to rising temperatures.
‘Blue’ carbon
Not just rainforests
Not only are we adding carbon dioxide to the atmosphere when we burn fossil fuels, but, when we clear forests for timber, for agriculture, or to make way for houses, we also reduce the capacity of our natural environment to take up CO2. It’s long been recognised that forests on land, such as rainforests, have an important part to play when it comes to mitigating the effects of global warming. That the health of our environment depends on the protection and regeneration of forests is a no-brainer. But they aren’t the only things to take in carbon dioxide and store carbon.
More and more, scientists have been recognising that oceans, and marine plants of coastal ecosystems, also play important roles as carbon sinks. In fact, research has revealed that they are even more efficient at sequestering carbon than forests on land. Even though plant biomass (organic matter) in the oceans is only a fraction of that on land, it cycles almost the same amount of carbon each year. What’s more, it can store carbon for extremely long periods of time.
The carbon sequestered by the ocean and the things living in it, and by marine plants of coastal ecosystems, has become known as blue carbon (after the colour of the ocean).
Oceans and marine organisms
Oceans cover more than two-thirds of Earth’s surface. Not only do oceans support a vast array of marine and plant life, but both the waters themselves and the things living in them are vital for removing carbon dioxide from the atmosphere.
How ocean waters capture carbon
Ocean waters cycle carbon through a mechanism known as the solubility pump. Carbon dioxide from the atmosphere dissolves in the salty ocean. The colder the seawater, the more carbon dioxide is dissolved. Because cold water is denser than hot water, it sinks, taking the dissolved carbon down into deeper layers of the ocean. It won’t stay there forever—eventually rising again to the surface in warmer areas, such as near the equator. However, this movement depends on the turning over of the ocean, which can take up to a thousand years.
Thanks to this process, it’s estimated that the ocean has absorbed around a third of all the anthropogenic (that is, caused by people) carbon dioxide emissions since the beginning of industrialisation. While the ocean’s capacity to absorb carbon dioxide is large (80 per cent of all the carbon released by the activities of humans will end up there), it’s a slow process. As we release more carbon dioxide into the atmosphere, and the surface of the ocean takes up more of it, the ocean is becoming more acidic—with dire consequences for many marine organisms and ecosystems.
How ocean plants and animals capture carbon
Living organisms in the ocean also capture carbon. Like the leaves of plants on land, phytoplankton (microscopic marine plants) and algae capture carbon dioxide through photosynthesis, keeping the carbon. Of all the world’s carbon removed by photosynthesis, 55 per cent is captured by the living organisms in oceans. Organic material that isn’t eaten by other ocean creatures sinks and, over time, a proportion of it accumulates on the ocean floor as sediment (matter that settles to the bottom of water), where it can be stored for centuries.
The capturing of carbon by living organisms happens both in open oceanic waters and, most effectively, in coastal environments (for reasons we’ll look at in a moment). In fact, the capacity for coastal marine vegetated habitats to bury carbon is estimated at around 180 times greater than that of the open ocean. The process by which living things in the ocean cycle carbon dioxide is known as the biological pump.
Coastal ecosystems
You mightn’t find muddy mangroves as sexy as lush green rainforests, but the ecosystems along the world’s coastlines are every bit as vital when it comes to removing carbon dioxide from our atmosphere. The maximum reported carbon sink capacity of salt marshes, for example, is more than ten times that of undisturbed Amazonian rainforests. Travel to Shark Bay, Western Australia, and you’ll see seagrass meadows extending over around 4,000 km2, which store a massive amount of carbon in their top metre—around 45 teragrams GLOSSARY teragramsa teragram (Tg) is a unit of mass equal to 1012 grams , which is around one percent of the total seagrass carbon stores worldwide.

There are three main kinds of coastal blue carbon sinks: mangroves, salt marshes and seagrasses. Like plants on land, they capture and store carbon in their living, above-ground biomass (stems, leaves and branches), in their underground roots, and in their non-living biomass (such as leaf litter or dead wood).
As well as this, coastal plants also capture carbon from sources outside their immediate environment. Carbon-rich particles from other organic matter are carried along in tidal waters and river runoff and are effectively trapped in the dense and complex vegetation along coasts where it's stored as sediment. It’s this ability to sequester carbon in sediment that makes coastal ecosystems particularly important as carbon sinks. Not only are they capturing carbon from areas beyond their immediate environment, but the carbon which is captured in sediment is stored for really long periods of time. While rainforests, for example, can sequester carbon for decades (or, at most, centuries), coastal blue carbon sinks can do so for thousands of years.
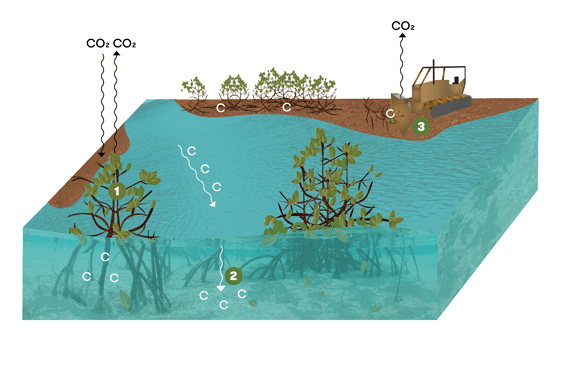
1. Carbon dioxide from the atmosphere is taken in during photosynthesis. Some carbon dioxide goes back into the atmosphere through respiration. The rest is stored as organic carbon in plant biomass and soil.
2. Carbon-rich particles from river runoff and tidal waters are trapped in mangroves’ dense vegetation and stored as sediment.
3. When mangroves are cleared or soil is dredged, carbon-rich sediments are exposed to oxygen in the atmosphere. This means the carbon is exposed to oxygen, forming carbon dioxide.
That’s because carbon in the comparatively well aerated soils on land eventually decomposes, releasing some back into the atmosphere. Coastal sediments, by contrast, are low in oxygen, which slows decomposition. They build up in layers, allowing more and more carbon to be stored. It’s estimated that those seagrass meadows in Shark Bay, for example, accumulated around 50 centimetres of soil every 1,000 years over the past 4,000 to 6,000 years. The thickness of organic mat escarpments there can reach up to 3 metres. And in Belize, Central America, there are mangroves with carbon-rich sediment more than 10 metres thick and estimated to be over 6,000 years old.
Feeling blue: coastal ecosystems under threat
Coastal development, overfishing, pollution, dredging and deforestation mean that the world is losing these important coastal habitats four times faster than its rainforests. Not only does the destruction of coastal ecosystems mean we have less carbon sinks but, when such carbon sinks are disturbed, the carbon stored comes into contact with oxygen, producing carbon dioxide, which then re-enters the atmosphere. It’s a double whammy.
About a third of the area covered by blue carbon sinks has been lost already and the rest is severely threatened.Blue Carbon 2009 UN Report
Of course, coastal ecosystems aren’t just important for dealing with carbon dioxide. Coastal vegetation acts as a natural barrier which protects communities from coastline erosion and extreme weather events such as severe storms. Coastal waters also provide local and international communities with food—supplying an estimated 50 per cent of the world’s fisheries. Mangroves are especially important as fish nursing-grounds. These important coastal areas need protection and regeneration—to ensure the safety and wellbeing of coastal communities, to protect the world’s food supplies, and to mitigate the effects of increasing levels of carbon dioxide in our atmosphere.





