Athelstan Laurence Johnson Beckwith 1930–2010

Written by Ian D. Rae

Athel Beckwith was an organic chemist whose research was concerned with free radicals, the reactive intermediates that play important roles in many organic chemical reactions. After studies and junior appointments at Australian universities, at Oxford University he worked with W. A. Waters and completed his doctorate at a time when scepticism about the very existence of free radicals was being rolled back by a small group of experimentalists. Returning to Australia, where he occupied chairs at the University of Adelaide and the Australian National University, Beckwith used studies of organic structure and mechanisms, revealed by kinetic methods and electron spin resonance spectroscopy, to become a world leader in this field of chemistry. He was honoured by election to Fellowship of the Australian Academy of Science (1973) and the Royal Society of London (1989), by several awards from the Royal Australian Chemical Institute, and by membership of the Order of Australia (2004). His extensive travels, often accompanied by his wife Kaye and their children, to work in overseas chemical research laboratories and to give presentations at international meetings, helped him to secure his place in networks at the highest levels of his profession. Several those who studied with him now hold important positions in Australian chemistry
Early Days
Athelstan Laurence Johnson Beckwith was born on 20 February 1930 in Perth, Western Australia, to Laurence Alfred and Doris (née Johnson) Beckwith. Later additions to the family were his brothers, Kingsley, born in April 1936, and Graeme Harvey, born on 17 April 1939. Doris was the daughter of a Perth cabinet maker, John Johnson, and his wife, Lilly. Laurence was born in Katanning, 200 km east of Perth in the wheat belt, where his father Alfred James Beckwith (1878–1951), a builder and carpenter, and mother Ethyl Blanche née Strutt, both Melbourne-born, had settled. Laurence moved to Perth at age twelve upon winning entrance to Perth Modern School, afterwards qualifying as a pharmacist and owning a business in central Perth. Looking further back (Beckwith 2003), Athel notes that of his great-grandparents three were Scottish, two English, one German and one Scandinavian, and that all had arrived in Australia in the mid-nineteenth century. He traced his English antecedents to seventeenth century Leeds and noted earlier manifestations of the Beckwith name in Yorkshire. It is attached to church bells and some thirteenth-century silverware in York Minster, and to the village of Beckwith shaw near Harrogate.
Both Laurence and Doris Beckwith were talented musicians. Laurence appeared frequently for the national broadcaster (Australian Broadcasting Commission) and sang with local choirs. The rich family life of music and reading was augmented by the presence in the Beckwith household of Doris’ parents who lived with them during the depression years of the 1930s. Grandfather Johnson was a strong influence on young Athel’s interest in carpentry and model-building, and in outdoor life that included time spent in the nearby bush, in fishing at Fremantle and swimming.
Schooling
The first few years were completed in Perth at Mt Hawthorn and Leederville primary schools, but in 1942 Athel with his mother, two brothers and grandmother were evacuated to the Porongurup area some 400 km south of Perth. Evacuations from actual and potential war zones took place under the aegis of revised regulations on national security and were implemented in the Northern Territory, Queensland and Western Australia. Athel attended Mt Barker school, undertaking his sixth-grade studies as a pupil in a combined class (grades 6–9) that enabled him to take his studies beyond those set down for primary school. He credited the excellence of his teacher, Mr Best, for the success of several Mt Barker students, Athel among them, who won scholarships to study at the state’s premier secondary school, Perth Modern, to commence their studies in 1943.
Things went well for a few months, in both academic and sporting fields (he was captain of the basketball team) but in April young Beckwith was struck down by an illness that kept him out of school until he was able to resume in 1945. The initial diagnosis was that extreme pain in his leg was caused by poliomyelitis, known as infantile paralysis because of its major impact on the group most vulnerable to this viral infection. This was an obvious diagnosis because at the time Perth was in the grip of one of three major epidemics that swept Australia in the middle years of the twentieth century.This was incorrect,however, and the mistake could have proved fatal. It was some months before the correct diagnosis of osteomyelitis, a staphylococcal infection of the bone, was made but by that time the disease was well advanced. The infection was treated with sulfa drugs and with the then-new penicillin, obtained for him by an American serviceman who was billeted with the family, but it was seven years before Athel was free of the bacterium. The result of the disease, the long convalescence and slow bone regrowth was that Athel’s knee and hip joints became fused, leaving him with a pronounced limp. Nevertheless, with his determination to make the most of life, he continued to walk, to hike the bush and to body surf as vigorously as possible.
While he was out of school Athel had read broadly and also studied by correspondence, and so subject to a performance review at the end of first term, he was allowed to retain his place with his erstwhile classmates. Short periods of hospitalization over the next two years did not affect his academic performance, although he never mastered Latin and dropped that subject as soon as possible.He completed his final (matriculation) year in 1947 with the rare feat of Distinctions in all seven subjects (Mathematics A, Mathematics B, Applied Mathematics, English, Physics, Chemistry and Music).
Athel had piano tuition from about the age of six, and in his mid-teens he added composition and then turned to jazz, a skill that greatly enhanced his social standing. A year or two later the sound of George Gershwin’s Rhapsody in Blue drew him to the clarinet, which he studied under a leading musician and played in the ABC Training Orchestra. His clarinet repertoire remained classical, and he played as a member of small groups over the next fifty years. On his first love, however, he was both a jazz and classical pianist (Figure 1).

Figure 1. Athel and grandson at the piano. University of Western Australia (UWA).
Beckwith’s interest in chemistry made his choice of undergraduate studies (1948–50) at the University of Western Australia (UWA) an easy one. In traditional fashion he enrolled in four subjects in first year (Chemistry, Physics, Mathematics and Zoology), three in second year (Chemistry, Physics and Mathematics), and two in his final year (Organic Chemistry and Inorganic Chemistry). The staff members of the Chemistry Department at UWA, whilst mostly Australian-born, had gained overseas experience and were equipped to pursue research in several fields. In addition, there was a flow of talented students, especially in chemistry, who were subsequently to occupy prominent positions in Australian science.
Athel’s main interest was in the field of organic chemistry—he liked preparative chemistry and he had enjoyed the lectures by Professor Doug White on organic natural products. In 1951 Athel began his Honours year with White, contributing to work on triterpenes in Australian native plants that was published in succeeding years (2, 6). When White went on sabbatical leave, Athel came under the supervision of another young staff member, Dr Joe Miller, who had introduced to the department the electronic theories then being developed in Britain by Robert Robinson and Christopher Ingold. This led to several joint publications (1, 3, 4, 5) exemplifying Miller’s interest in nucleophilic substitutions at electron-deficient aromatic carbons, and saw Athel graduate in March 1952 with First Class Honours in organic chemistry.
Rather than proceed to PhD studies that were then becoming available in Australia (Rae 1999), Athel took a position as Graduate Assistant in the chemistry department and also enrolled in a Master of Science degree to be completed by research. His departmental duties included some lecturing and a good deal of laboratory supervision, but allowed time for research. His research concentrated on the reactions of diazonium salts with various substances, important steps in the production of many dyestuffs, but his attempts to elucidate the reaction mechanisms via study of their kinetics came to grief over unexplained variability in the observed reaction rates. ‘I couldn’t understand what was going on’, Beckwith said, and ‘later I returned to this problem’. Work with PhD student Gordon Meijs on cyclization reactions of diazonium salts, which were later shown to have considerable synthetic potential, probably represents the return to this subject (103, 139, 143).
As the year progressed, Athel made plans to take up a scholarship made available under the Hackett Bequest to UWA for him to study for the PhD degree in London with Professor Derek Barton. He changed direction, however,when the head of chemistry at the University of Adelaide, Professor A. Killen Macbeth, contacted him via the head of the UWA department, Professor Noel Bayliss, and asked him to come to Adelaide as junior lecturer. Athel and Kaye Marshall had become engaged in the Spring of 1951, and in January 1953, a few days after her twenty-first birthday, they were married and after starting their honeymoon in Perth they set off a week later for Adelaide, on the M.V. Westralia. Athel and Kaye shared interests in books, music and the outdoors and their wedding marked the formal beginning of a partnership in which Kaye developed her own career while playing an important adjunct role in Athel’s (K. Beckwith 1999).
Beckwith’s teaching duties in Adelaide were not in organic chemistry, but in inorganic and physical chemistry, where he noted that he managed to stay ‘one lecture ahead’ of his students. In his research he explored mechanisms of reactions and also did some natural product work and synthesized thyroxine analogues. In October he wrote to UWA requesting that his candidacy be transferred from MSc to PhD and this was granted the following month, with G. M. Badger, who had recently arrived in Adelaide (Rae 2009), suggested as a possible supervisor. The thesis topic was to be, echoing Miller’s influence and Beckwith’s interest in electronic theory, ‘Some Aromatic Nucleophilic Displacement Reactions’.
The overseas doctorate still beckoned, how- ever, leading to a successful application for a Commonwealth Scientific and Industrial Research Organisation (CSIRO) overseas scholarship. Beckwith’s aim was again to work with Barton on natural products, but the Chief of the CSIRO Division of Industrial Chemistry, Ian Wark, thought there might be a better, more adventurous alternative, and approved of Beckwith’s second suggestion that he should work with W. A.Waters, an expert on free radical chemistry, at Oxford University. This interest in free radicals had its roots in the erratic kinetic results obtained in Perth and it led to more than fifty years of research for Athel Beckwith.
Oxford
Athel and Kaye and baby daughter Catherine Louise (born October 1953) travelled by ship to England on the S.S. Orcades, arriving in London in November 1954 and proceeding to Oxford a week later. At that time only a few chemists were working on free radicals and many, including the doyen of organic chemistry, Robert Robinson, did not believe in them. However, after initially deriding chemists like Waters who had staked their careers on the existence of the highly reactive and therefore fleeting entities, Robinson was unusually magnanimous in stating that they had been right all along (Norman et al. 1986). Waters was a gentle supervisor who encouraged his students to develop their ideas and reserved Saturday mornings to visit the laboratory and discuss progress with them. Four publications (7–10) resulted from Beckwith’s studies of the attack of radicals on aromatic systems. A fellow student, R. O. C. (‘Dick’) Norman (later to be UK Chief Scientist) was co-author on one of these papers and he became the first of a ‘family’ of free-radical chemists with whom Beckwith developed strong personal and professional ties. Both contributed to a Chemical Society publication in 1970 marking Waters’ retirement (52).
Although Athel was attached to Balliol College, dining in twice a week, the family had to live out. The scholarship did not support more than basic living and so the Beckwiths took in one or two students as lodgers, an arrangement that required Kaye to take out a licence as a boarding house keeper. As well as providing rental payments, the students helped with babysitting, and so enabled the parents to take part in the rich social life of the Oxford community. Their social circle included other Australians there at that time, among them R. J. (‘Bob’) Hawke and his wife Hazel. Bob was a friend from Athel’s primary school days who had been a year ahead of him at Perth Modern and was to serve as Prime Minister of Australia 1983–91.
Prior laboratory experience, typical of Australians proceeding to postgraduate work in Britain following Masters degrees or other research experience, enabled Beckwith to complete his D. Phil. degree within two years and see it conferred in October 1956. The family despatched most of their goods and moved to London in November in readiness for the return journey to Australia, only to learn that their ship was trapped in the Red Sea by the crisis that followed British, French and Israeli attempts to regain control of the Suez Canal from Egyptian authorities. CSIRO extended their scholarship support and the Beckwiths made the best of their time in the capital that freezing cold winter. The entertainer Rolf Harris, another Perth Modern acquaintance, visited them from time to time to share the warmth of their oven and the family became adept at finding warm places, among which were art galleries and the hothouses at Kew Gardens.
While they waited for a passage home, Athel continued experimental work at Oxford with Waters and Norman and writing at home in London, and he was grateful to accept an invitation from the Chemical Society to present the results of his Oxford work at a conference in London where, for the first time, he addressed a high-level audience. Eventually, in February 1957, they were able to board the S.S. Strathaird for a homeward voyage around the Cape of Good Hope, but there were further delays when the ship broke down in Cape Town and the necessary repairs there and in Durban delayed them for a week. They did not waste the opportunity to explore the hinterland but found the Apartheid regime everywhere oppressive.
Return to Melbourne
The Beckwiths arrived in Melbourne in early 1957. Life was pleasant there and the pace of research at CSIRO was leisurely. Kaye wrote that ‘for the first and only time in my married life I had a nine to five husband’, although things got busier when their second child, Paul, was born in September 1957. Beckwith had joined the research group of H. H. Hatt at CSIRO’s Fisherman’s Bend laboratories, where the aim of the research was to find uses for wool wax. Athel was assigned the task of functionalizing the main component, lanosterol, with a view to converting it to possibly useful chemical substances. Background reading revealed that a related but better-known sterol, cholesterol, became oxidized during storage and that a new hydroxyl group was introduced into the hydrocarbon side-chain, remote from the only sites of chemical activation, the hydroxyl group at the 3-position and the C5-C6 double bond. Beck- with’s attempts to oxidize cholesterol in solution did not produce the diol, 25-hydroxycholesterol, but oxidation of the crystalline solid suspended in water or spread on a glass plate did so. Beckwith ascribed this selectivity to the way cholesterol molecules were packed into the crystals so that the side-chains were exposed on the surface, as had been demonstrated by X-ray crystallography.
The work was published the following year (11), by which time Beckwith had taken up a lectureship at the University of Adelaide, and it was some years before he returned to the matter of remote functionalization in steroid molecules. He noted that the conversion of readily available cholesterol into steroid hormones proceeded by degradation of the side-chains of molecules in which the cholesterol functional groups were protected against oxidative attack. In Beckwith’s hands, the reaction of chromic acid with 5α,6β-dibromocholestan-3β-yl acetate, followed by debromination and hydrolysis, gave a 2% yield of the 25-hydroxy derivative which he proposed was the initial product from which further oxidation products, isolated by him and other workers, were formed (17).
Beckwith’s first research at Adelaide was similar to that of his Oxford work, producing a series of papers on oxygen-centred radicals (12, 13, 18, 19, 28), but he also continued to work on aromatic substrates (15, 20). He reviewed the field (14) before exploring analogous reactions with ferrocene (21, 25, 26, 34, 35) and return- ing to this old interest from time to time (36–39) (Figure 2).
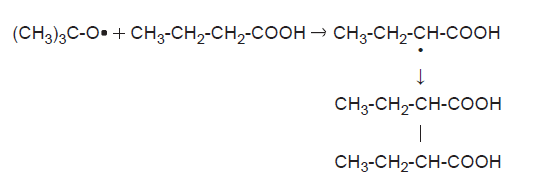
Figure 2. Free radical attack on butanoic acid (12).
Beckwith’s colleague and head of depart- ment, Professor Geoffrey Badger, was interested in the ability of polycyclic hydrocarbons to induce animal cancers, and this led Beckwith to speculate about possible chemical pathways through which this carcinogenesismight be acti- vated. Thinking, naturally, of free radicals, he investigated the reactions of sulfur-based radi- cals with aromatic substrates and found a rich field of chemistry (16, 22, 24, 27). Even though naturally occurring thiols such as glutathione and cysteine took part readily in such reactions, the carcinogenesis hypothesis could not be confirmed and the work was abandoned although sulfur-based radicals were later found to be useful in organic synthesis.
In 1960 the Royal Australian Chemical Institute awarded Beckwith its Rennie Medal (named after the first professor of chemistry at Adelaide) for the most meritorious research by an Australian chemist under thirty years of age. At the end of the year he was promoted to Senior Lecturer and three years later to the rank of Reader. Beckwith had not forgotten his earlier desire to work with Professor Derek Barton at Imperial College, London, who at the time was engaged in research on remote functionalization of the kind that Beckwith had explored with cholesterol. So, supported by a travel grant from the British Council under the Commonwealth Universities Interchange Scheme, he took sabbatical leave from Adelaide to spend a year in London. Following a brief holiday in Perth with family, the family of four sailed from Fremantle in the S.S. Strathnaver on 24 January 1962 and spent the year in London, suffering together through the cold winter of 1962–63 and the last of the London smogs. Barton’s group had been unsuccessful in achieving remote attack by oxygen-based radicals generated from hypoiodites, but Beckwith found that ultraviolet irradiation of N-iodo-amides, and hydrolysis of the resulting γ-iodoamides, produced the desired γ-lactones (23, 29).
Beckwith’s time in London was extended because the first two months had been lost while he underwent back surgery. He had experienced pain before leaving Adelaide and had the good fortune on the voyage to Britain to encounter a fellow passenger, a neurosurgeon, who diagnosed spinal degeneration and arranged treatment for him in London.Thus it was not until 14 February 1963 that the family embarked on the S.S. Himalaya to travel via the Suez route and arrive home a month later. As well as the experience of working in Barton’s laboratory, Beckwith had renewed old acquaintances in Britain and also attended the 1962 IUPAC Symposium on Natural Products Chemistry in Prague. As well, he had visited the Technical University of Eindhoven to learn about mass spectrometry and he had discussions with chemists at the National University in Singapore, on his way back to Australia.
Back in Adelaide, Beckwith found that γ-lactones could also be formed from N-chloroamides (31), but that oxidations of carboxylic acids gave only poor yields of lactones (32). Turning to other reactive nitrogen species, he and his students found that the oxidation of primary amides generated nitrene species R-CO-N: that rearranged to isocyanates and these could be trapped as acylamines (30). This was reminiscent of the well-known Curtius reaction in which the nitrene was generated by thermal decomposition of acyl azides.There followed a series of papers on oxidations by lead tetra-acetate (33, 36, 37, 38, 40, 41, 42). The strength of the Beckwith group was by that time attracting graduate students and more senior researchers such as Professor W. B. Renfrow from Oberlin College, Ohio. Renfrow and his wife Antoinette (Toni) both worked in the laboratory and together with graduate student Jillian Teubner were co-authors on published work (43).
The year 1964 was an eventful one, first on account of the birth of the Beckwiths’ third child, Claire, and then the resignation of Professor Geoffrey Badger to take an executive position with CSIRO, from which he was not long afterwards to return to the University and become Vice-Chancellor. Athel applied for the Adelaide chair, as he did for a corresponding chair at the UWA, and there was a delay while the institutions decided on their respective appointments until in February 1965, at the age of 35, he was able to take up the position of Professor and Head of the Department of Organic Chemistry at the University of Adelaide. One of his first actions was to appoint members of staff to a departmental committee but, with the democratic movement of the late 1960s and then the 1970s still some years away, the committee’s role was always advisory and the Head continued to take responsibility for decisions.
Under the generous conditions prevailing at the University of Adelaide, Beckwith was able to take sabbatical leave again in 1968. By then Kaye’s environmental activism—opposition to development in ‘green’ zones—had led to her election as Councillor in the City of Mitcham, but she took leave and the whole family travelled by ship, the S.S. Himalaya, leaving Adelaide on 6 January 1968. Athel had arranged to work with his fellow-student of Oxford days, Dick Norman, who was by then Professor of Chemistry at York University. The particular focus of Beckwith’s study programme was electron spin resonance (ESR) spectroscopy. Energy at microwave frequencies could be absorbed by the unpaired electrons of free radicals when the species were held in a strong magnetic field, and less than two decades from the time when most chemists did not believe that free radicals were important reaction intermediates, commercial instruments were becoming available that were suitable for routine use by organic chemists (Figure 3).

Figure 3. Beckwith at the ESR spectrometer.
The major features of the ESR spectra that they obtained were the fine splitting on the ESR sig- nals, which revealed the relationships between the free electrons and nearby nuclei such as those of hydrogen and nitrogen atoms, the size of the fine splitting being generally related to the number of intervening chemical bonds.
While in the UK Beckwith took the opportunity to visit other research laboratories and also wrote up a good deal of Adelaide work that was published later that year. In October 1968 the Beckwith family travelled to Canada and the USA, where Athel, with support from the Carnegie Foundation, undertook a gruelling lecture tour—thirty universities in three months— during which he made some important contacts. One was with Professor Cheves Walling, doyen of American free radical chemists, and the other was Dr Keith Ingold at the National Research Council of Canada. Beckwith and Ingold visited each other on several occasions, remained close friends and shared several research publications (81, 90, 91, 122, 183, 205).
Working together at the University of York in 1968, Beckwith and Dick Norman had found new ways to generate alkyl and aryl free radicals within the cavity of an ESR spectrometer, and their results were reported early in the following year (45, 46). Large, expensive scientific instruments such as nuclear magnetic resonance (NMR) and ESR spectrometers and mass spectrometers were becoming essential to the practice of organic chemistry.Adelaide’s first NMR spectrometer was installed in 1962 and in May 1966 Beckwith hadmade a three-week visit to the Hitachi company in Japan to learn about the operations of the mass spectrometer that was soon to be delivered to Adelaide.While he was at York it became clear to Beckwith that ESR was also a technique to which he needed ready access in his laboratory, and so one of the first things he did upon returning to Adelaide in early 1969 was to approach the Australian Research Grants Committee for funding. Fortunately, there were at the time uncommitted funds that had to be expended by the end of the financial year on 30 June and soAthel got his spectrometer promptly. The first results, describing aryl radical cyclizations, were obtained by graduate student W. B. (Bill) Gara and published as research notes that same year (47, 48), with full papers following some years later (69, 70).
One of the lead tetra-acetate reactions (42), in which pyrimidinediones were produced, attracted the attention of the Maumee Chemical Co., a US manufacturer of heterocyclic compounds and part of the Sherwin Williams group, the interests of which lay in paints and other surface coatings. They contacted Beckwith in early 1969, offering to pay the cost of relevant patents that Beckwith would then assign to them in return for payments to the University. The Australian market for such chemical substances was small but world-wide demand was likely to be sufficient that an international manufacturer could benefit from them, and so the University allowed patenting to proceed. A German patent, No. 1926475, was granted in December 1969, naming Beckwith as inventor. Subsequent US patents, Nos. 388750, 3947416 and 3947442, were granted to Beckwith in 1975 and 1976 and assigned to the Sherwin Williams Co. Alater review(Coppola 1980) described the lead tetraacetate oxidation as ‘the method of choice’ for production of such compounds, and another (Kappe and Stadbauer 1981) noted that these companies had patented several discoveries in this field of chemistry. In 1985 the patents were reassigned to the PMC Specialties Group Inc. but their coverage has since expired. In 1968, however, Students for Democratic Action, a radical group at the University of Adelaide, accused the University and Beckwith in particular of working for the US ‘military industrial complex’. There ensued demonstrations at meetings of the University Council—where the matter was listed in confidence as ‘agreement between Professor Beckwith and an un-named American company’—and in the Department of Organic Chemistry, during which Beckwith was defamed and several chemistry students were arrested. The fact was that Maumee intended to use the chemicals as corrosion inhibitors and for ultra-violet protection, certainly not the herbicides then being used by American troops in Vietnam that were in the students’ minds. The fuss eventually subsided and harmonious relations were restored.
The years 1970–75 marked a productive period for Beckwith’s group, and Athel was elected to Fellowship of the Australian Academy of Science in 1973. In his laboratory, ESR spectroscopy became a customary tool (50, 54, 57, 60), while kinetic methods were often used to study reaction mechanisms, especially where rearrangements were involved (51, 53, 56, 58, 59, 65). Beckwith was a recognized expert in rearrangements and published a succession of reviews on this topic (52, 55, 90). The development in the late 1960s of the principle of conservation of orbital symmetry and adumbration of the ‘Woodward-Hoffmann Rules’ by the chief protagonists led to great interest among organic chemists. Although orbital symmetry never played a big part in Beckwith’s analysis of reaction mechanisms, he did explore ideas of stereo-electronic control, beginning in 1971 (53) and continuing for many years (62, 70, 71, 86, 88, 96, 101). Beckwith was assisted in coming to an understanding of the principles by a leading Australian theoretical chemist, Leo Radom, and the two shared authorship of some articles (117, 219). In 1980 and 1981 Beckwith published guidelines on these aspects of radical reactions (94, 101), and thesewere often referred to as ‘Beckwith’s rules’ (Figure 4).
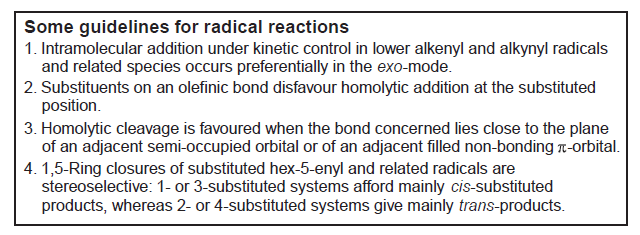
Figure 4. Beckwith’s Rules (94).
In Adelaide Beckwith had acted as consultant to several small companies. Then in 1972 he accepted an invitation from the Chief of CSIRO’s Division of Applied Chemistry in Melbourne, Dr David Solomon, to be a consultant to the organization’s work on copolymerization of allylamines that were to be used in the Sirotherm ion-exchange resins (Spurling 2011). Beckwith devoted one day a month to this activity, in return for a modest consulting fee (subsequently used to facilitate conference travel) and rights to co-authorship that yielded a series of published papers (67, 68, 77) covering practical and theoretical aspects of free-radical cyclopoly- merizations, especially of diallylamines from which industrially important polyamines could be formed. Later he was to consult on polymer chemistry for the paint manufacturer, Dulux, where his ideas were influential in the company’s development of new products; he was also involved in other collaborative work with CSIRO researchers (109).
Beckwith was aware that several enzyme mediated chemical changes in natural systems involved attack at apparently unactivated positions in molecules. Some free-radical reactions have this characteristic, and Beckwith speculated that free radicals might be involved in the natural systems. He pursued this interest by taking a year’s leave from Adelaide to work at Oxford with Professor Sir Ewart Jones, an expert in biological chemistry. While daughter Cathy stayed behind in Adelaide to study for her B.A., the rest of the family set off in March 1974, flying first to Athens and visiting several other European scientific centres of chemical research on their way to England. During the year Athel was able to visit North America to attend the Gordon Conference on Free Radicals and to visit Keith Ingold in Ottawa to initiate collaboration that involved work on free radical chemistry. Returning to Australia a year later, the family travelled via Moscow and Singapore where again Athel met with chemists. During the year he lectured at several UK and European universities and attended conferences, including the International Conference on Free Radical Chemistry, in Italy, where he chaired a session. The involvement of free radicals in enzymic reactions could not be demonstrated and this outcome, plus the lack in Adelaide of the microbiologist collaboration that Jones enjoyed at Oxford, meant that there was no direct follow-up when Beckwith returned to Adelaide.
However, thinking about remote functionalization rekindled Beckwith’s interest in reactions taking place at crystal surfaces, which he had investigated in the case of cholesterol many years before. The result was several articles reporting reactions of ozone with solid substrates (80, 82, 85, 87). Later, the biological interest led to a study of free radical formation in vivo from the anaesthetic halothane (105). Beckwith’s continuing interest in possible involvements of free radicals in biological systems led him in 1979 to embark on an eight-month period of study leave, taking Kaye and their two younger children, to work at Oxford with Professor Jack Baldwin. A joint publication arose from that period (100) and was followed up in Adelaide with other studies. These were the formation of a β-lactam in a free radical reaction that demonstrated a possible biosynthetic route to penicillin (113), and synthesis of other β-lactams in fused ring systems (121).While he was away, Beckwith gave invited lectures at several universities and research centres in the UK, France and Israel, and attended the Euchem Conference on Free Radicals, which had become a major venue for presentation of his work.
It was not all chemistry in Adelaide. Both Athel and Kaye were members of the Southern Jazz Club and frequently attended its Thursday night band concerts in the Flagstaff Hotel, and Athel played with the band at the concert they held to farewell the Beckwiths from Adelaide. Kaye was active in the environment movement, and both of them were involved in promoting social justice for the local aboriginal community. Leaving Adelaide was hard for Kaye, who had advanced in her career in municipal government to be a Councillor and then an Alderman, but after two decades in Adelaide Athel was ready for new challenges and opportunities.
Canberra and the Australian National University
Following Professor Arthur Birch’s retirement from the Research School of Chemistry at the Australian National University (ANU) in 1980, Athel Beckwith and Lew Mander were appointed to professorships, Athel taking up his appointment in mid-1981. Among the advantages he found in Canberra were better equipment, including ‘a new ESR spectrometer that was much more powerful than the one in Adelaide’, and ‘a large number of highly proficient and extremely helpful technical staff’. During his ANU years, Beckwith was to benefit substantially from this technical assistance, notably through the work of Dr Tony Willis, a crystallographer who joined the school in 1985. X-ray crystal structure determinations (176, 179, 185, 203, 204) confirmed structures that helped to elucidate reaction mechanisms. Another important contributor was Dr Steven Brumby who did much of the ESR spectrometry for the group and conducted research on the analysis of ESR spectra (146–148, 187, 189, 197).
Freed from undergraduate teaching and with the strong support that ANU provided, Beckwith was extraordinarily productive at the Research School of Chemistry. The chemistry of free radicals uncovered by him and others had not only gained acceptance for radicals as real species that played important parts in the mechanisms of many well known reactions, but the understanding of how to generate and use free radicals had developed to the point where radical reactions entered the organic chemist’s ‘tool box’. The propensity for formation of five-membered rings was put to advantage by Gilbert Stork (Stork and Baine 1982; Stork and Mook 1983) and by Curran (Curran and Rakiewicz 1985a, 1985b), who synthesized fusedring triquinane natural products. Stork covered some of this chemistry when he delivered the 1987 Birch Lecture ‘Radical Cyclisation in Natural Product Synthesis’ at ANU, and a later review showed how synthetic chemists had made good use of free radical chemistry in the ensuing decade (Jasperse et al. 1991).
During his Canberra years Athel became more deeply involved in the work of the Australian Academy of Science, serving as a Council member (1983–86), Vice-President (1985–86) and Treasurer (1997–2001), and he was also a member of a panel appointed to review the funding of research in organic chemistry in Australia (227). Kaye gave time to the Women’s Electoral Lobby and served as Administrator for the Citizens Advice Bureau. Their daughter Cathy married Martin Banwell, an organic chemist who was later to become Athel’s colleague at ANU, while son Paul completed his D. Phil. at Oxford, working with Jack Baldwin. In the later 1980s Athel served as a consultant to ICI Australia, which maintained a research laboratory in that period but abandoned it in the 1990s. His advice was sought on a range of chemical projects but especially on the search conducted for novel herbicides (Watson 2011). Consulting work with the ICI Australia subsidiary, Dulux, also continued.
In Canberra Kaye deepened her interest in Aboriginal art and held positions in environment groups. Despite these local involvements she was often free to join Athel as he continued to travel extensively to present research results at conferences and to visit other laboratories where he was able to learn of recent or forthcoming developments and to engage in discussion with other free-radical chemists. From his earliest days as a research leader, he was always ‘hands on’. Just as sabbatical leave at York had enabled him to develop methods that he was to take home to Australia, a period spent with Professor Alwyn Davies in London allowed him to begin work with molecular mechanics calculations that were then developed in Canberra (119, 125). Other numerical methods came later (146, 147, 163 198, 210, 219) (Figure 5).

Figure 5. Beckwith and David Harman performing a molecular mechanics calculation.
In research at Adelaide and Canberra, free radicals were generated by various means (136), including photolysis and oxidation by copper (II) ions, but greatest use was made of stannyl radicals that came into widespread use after 1975 (Neumann 1987). Chain reactions with these metal-centred radicals, initiated by fission of azobisisobutyronitrile (AIBN) in the presence of tri-n-butyl tin hydride, proceeded by abstraction of halogen atoms from suitably constructedmolecules, or interactionswith other heteroatoms, to generate specific carbon-centred radicals. From the beginning of his Canberra period, although he did not attempt complete syntheses, Beckwith explored free-radical reactions that could be used to construct key sections of the molecular frameworks of natural products.These were inevitably cyclization reactions, which Beckwith explored using kinetic, X-ray crystallography, ESR spectroscopy and theoretical tools.Cyclizations were the subject of amajor group of research publications (113, 119, 121, 125, 127, 132, 133, 134, 137, 141, 145, 151, 158, 161, 167, 168, 170, 172, 177, 178, 181, 182, 192, 202, 208, 214, 215, 217, 220) and reviews (136, 152).Molecular rearrangements and other chemical reactions were frequently the subject of reaction rate studies, including the determination of absolute reaction rates, and data from the Beckwith group were reported in three major contributions to the Landholt-Börnstein series (116, 197, 224) edited by one of the pioneers of free-radical chemistry, Hanns Fischer, who spent three months in Canberra in 1992. Beckwith and Ingold memorialized him when he died in 2005 (223).
In 1989 Beckwith was elected a Fellow of the Royal Society of London, and when his colleagues Professor Lew Mander (1990), John White (1993) and Martin Bennett (1995) were elected, there were seven FRSs on the staff of ANU’s Research School of Chemistry, the others being Birch (1958), Craig (1968) and Sargeson (1983). In 1993 Beckwith travelled to Britain to deliver a Centenary Lecture for the Chemical Society (190) and to receive the Centenary Medal. A period spent in Germany in the 1990s where Beckwith was supported by a Humboldt fellowship enabled him to work in the laboratories of Christoph Rüchardt at the University of Freiburg, where ESR spectroscopy was applied to the measurement of bond dissociation energies and stabilization energies (211).
The Beckwith Chemical ‘Family’
Co-authorship of research publications shows an important aspect of Beckwith’s status as a scientist. There were early papers on which he was not the lead author and, as is the cus- tom in the sciences but not the humanities, he shared authorship with graduate students and postdoctoral fellows who had worked under his direction. However, he also collaborated on equal terms with several leading organic chemists from around the world. I have already described joint work with R. O. C. Norman (45, 46), D. H. R. Barton (23, 29), K. U. Ingold (81, 90, 122, 183, 205, 223) and A. G. Davies (153, 165) but collaborations arose in other ways, too. French chemist Bernard Maillard, for example, first met Beckwith in Ingold’s Ottawa laboratory, and later shared preliminary results with him that led to joint publications (208, 215).
As the fame of the Beckwith group grew, scientists came to spend periods of leave working there, and often brought their families to this (for some, faraway) centre of chemical excellence. This accounted for collaborations with the Renfrows (43), Swee Hock Goh (110, 111), Warkentin (150), and Dyall (168). An especially interesting case was that of Stephen Glover, a South African who completed his PhD degree working with André Goosens, who had worked with Barton and Beckwith at Imperial College (29). After a period of leave spent in Canberra with Beckwithin 1984, Glover emigrated to Australia and took up a position at the University of New England, in Armidale, in gaining which he credits Beckwith’s influence. Glover later spent a period of leave in Canada, at McMaster University with John Warkentin, whom he had met while the two were guests in Canberra a decade earlier. Another whose position in Beckwith’s laboratory resulted in emigration to Australia was Anil Abeywickrema, a Sri Lankan chemist who had completed a PhD at Flinders University in Adelaide. Abeywickrema returned home to a position at the University of Colombo but remained in Australia after his subsequent post-doctoral experience in Canberra (122, 131, 134,139, 144, 145). Those who made several visits include Goh of the University of Malaya, Dyall from Newcastle University, and Andreas Zavit- sas of Long Island University (140, 163, 198, 210, 225). Israeli chemists Y. Mazur and Morde- cai Ruben exchanged visits with Beckwith, with Rubin spending several periods in Canberra.
Students and postdoctoral fellows moved between groups, such as Dean Struble who worked with Beckwith in the late 1960s (44, 56) and also with Barton, finally returning to Canada to forge his career. They formed connections between the groups and sometimes brought their erstwhile research directors into collaboration. Such was the case with Ian Davison, a Davies student (153, 155) who was later a postdoctoral fellow in Canberra (191, 195), and Gordon Meijs, a Beckwith PhD from Adelaide days who subsequently spent a postdoctoral period with J. F. Bunnett in Santa Cruz and brought together Beckwith’s and Bunnett’s mutual interest in SRN 1 reactions (130). In the early 1980s, Beckwith was a visiting lecturer at Duke University, North Carolina, where his host, Professor Bert Fraser-Reid invited him to comment on some unusual aspects of the photochemical addition of alcohols to enones in the presence of benzophenone. Beckwith established that this involved hydrogen abstraction followed by conjugate addition of the resulting hydroxymethyl radical, bringing understanding of the stereoelectronic control when cyclic enones were involved (156).
The Australian Journal of Chemistry pub- lished an issue to mark Beckwith’s 65th birthday in February 1995. In his introductory remarks, Beckwith’s graduate student and later colleague at ANU, Chris Easton, outlined Beckwith’s career and his achievements and noted that his work had been cited over 1000 times in 1992–93. He also spoke of the support that Athel and Kaye gave to students and colleagues (Easton 1995). The issue included papers from chemists from around the world who had associations with Beckwith’s research. In the gracious introduction to his paper, Professor Gerald Pattenden, University of Nottingham, commented that ‘few would argue with the leading position that AthelL. J. Beckwith occupies in the line of pioneers of the application of free radicals in contemporary organic synthesis. The plethora of citations and publications from his laboratory are ample evidence of the ingenuity and creativity of his contributions in this area’ (Pattenden 1995). Beckwith and his inorganic chemistry colleague, Alan Sargeson (Bosnich 2011, 2012), having reached the statutory age of 65 years, retired at the end of 1995 and a symposium to honour their achievements was held in Canberra in January 1996. In February 2010 a symposium entitled ‘Perspectives of a Radical Chemist’ was held at ANU to honour Athel’s 80th birthday. It featured lively presentations from former students and collaborators.
The Royal Australian Chemical Institute, of which he was President in 1984–85, had recog- nised Beckwith’s contributionsto chemistry with the award of their H. G. Smith Memorial Medal in 1980 and the Organic Division Medal in 1992 and its highest award, the Leighton Memorial Medal, in 1997. In civil life, he was awarded a Centenary of Federation Medal by the Australian Government in 2001, and in 2004 became an Officer of the Order of Australia (AO).
Tributes
Athel Beckwith was killed in a car crash on 15 May 2010. He is survived by his wife, Kaye, who was badly injured in the crash but recovered after hospitalization, and by their three children, Paul, Cathy and Claire. These three and Beckwith’s brother Graeme spoke at the subsequent celebration of his life, as did several ANU colleagues. Claire’s contribution was a reading from Tolkien’s masterpiece, The Lord of the Rings, which Athel adored and had read as a serialised bedtime story to each of the children. The music reflected Beckwith’s tastes and abilities— a Mozart clarinet trio and Gershwin’s Rhapsody in Blue and Summertime—while Tom Lehrer’s The Elements served to underscore his love of chemistry.
In 2011, almost a year after his death, the Australian Journal of Chemistry again dedicated an issue to Beckwith, with articles from a range of Australian and international chemists. The guest editor was Peter Duggan, who had com- pleted his PhD with Beckwith at ANU in 1990. In his thoughtful introduction (Duggan 2011) Dug- gan mentioned the ‘reaction that Athel would ultimately become best known for—the cycliza- tion of the hex-5-enyl radical’ (Figure 6)—and the ‘Beckwith Rules’ (94).
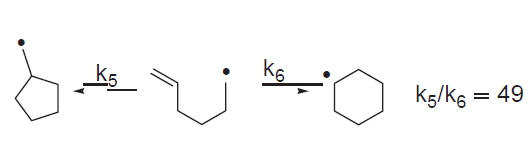
Figure 6. Cyclization of the hex-5-enyl radical (88).
A reflective paper by Algi Serelis, a Beckwith postdoctoral fellow from his late Ade- laide period, recounted the way that Beckwith developed a deep understanding of free radi- cal reactions and developed them as tools for the synthesis of polycyclic structures (Serelis 2011). Former Beckwith student Carl Schiesser, now a professor at the University of Melbourne, organised a themed issue on free radical chemistry in memory of Athel Beckwith in the journal Organic and Biomolecular Chemistry that attracted 48 articles (http://blogs.rsc.org/ob/category/web-theme- issues/, accessed September 2011). In an article of which Beckwith was named as co-author, Schiesser wrote about the ‘dark ages’ in which ‘free radicals remained largely inaccessible to synthetic chemists’ and the ‘renaissance period’ beginning in ∼1970 when key questions wereasked and answered, and free radicals became useful and not ‘poorly understood curiosities, often scapegoats for unwanted outcomes’ (226).
Early in 2011 the Organic Division of the Royal Australian Chemical Institute renamed their annual lectureship for recently appointed staff in his honour. Funding is available for the Athel Beckwith Lecturer to deliver six lectures in state capitals or major regional centres during the year of the lectureship. His continuing influence on chemistry in Australia is perpetuated by the Centre of Excellence for Free Radical Chemistry. This dispersed organization, based in several Australian universities, includes several Beckwith graduates, one of whom is the Centre Director, Professor Carl Schiesser of the University of Melbourne, and another, Chris Easton, a professor in the Research School of Chemistry at the Australian National University.
A leading historian of chemistry, Colin Russell, has described how free radicals joined ion pairs and heterolytic bond cleavage in the chemical orthodoxy by about the middle of the twentieth century (Russell 2004),andhecredited D. H. Hey, W. A. Waters, D. H. R. Barton and C. Walling with seminal contributions to the field. Athel Beckwith’s important role was described by Keith Ingold in a moving eulogy delivered at the opening of the EUCHEM meeting on free radicals that was held in Bologna just a few weeks after Athel’s death (Ingold 2010). In it, he described Beckwith’s chemistry, alluded to their long friendship and collaboration, and described Athel as ‘an outstanding scientist’ and ‘one of the great chemists of the twentieth century’. A tribute that pointed to broader interests in life came from the Canberra Jazz Club, who remembered him as ‘the chemistry professor who took on free radicals—and won’, a quotation from an obituary that first appeared in the Sydney Morning Herald in June 2010.
Bibliography
- A. L. Beckwith, J. Miller and G. D. Leahy (1952). The SN mechanism in aromatic compounds. Part III. J. Chem. Soc., 3552–3556.
- J. R. Anstee, H. R. Arthur, A. L. Beckwith, D. K. Dougall, P. R. Jefferies, M. Michael, J. C. Watkins and D. E. White (1952). West- ern Australian plants. Part VI. The occurrence of betulic, oleanolic, and ursolic acids. J. Chem. Soc., 4065–4067.
- A. L. Beckwith and J. Miller (1952). On N-(2,4- Dinitrophenyl)phthalimide. Aust. J. Sci. Res., Ser. B 5A, 786–789.
- A. L. Beckwith and J. Miller (1954). The SN mechanism in aromatic compounds. IX. Some reactions of polynitrodiaryl ethers. J. Org. Chem. 19, 1416–1423.
- A. L. Beckwith and J. Miller (1954). The SN mechanism in aromatic compounds. XI. Some reactions of aminodinitrodiaryl ethers. J. Org. Chem. 19, 1708–1715.
- A. L. Beckwith, A. R. H. Cole, J. C. Watkins and D. E. White (1956). Triterpenoid compounds. III. Phyllyrigenin. Aust. J. Chem. 9, 428–431.
- A. L. J. Beckwith and W. A. Waters (1956). Reactions of anthracene and 9-methylanthracene with the free radicals derived from di-tert.-butyl peroxide. J. Chem. Soc., 1108–1115.
- A. L. J. Beckwith and W. A. Waters (1957). Reac- tion of anthracene with benzyl radicals. J. Chem. Soc., 1001–1008.
- A. L. J. Beckwith and W. A. Waters (1957). Reaction of chlorobenzene with methyl radicals. J. Chem. Soc., 1665–1668.
- A. L. J. Beckwith, R. O. C., Norman and W. A. Waters (1958). Free-radical reactions of 9: 10-diphenylanthracene. J. Chem. Soc., 171–175.
- A. L. J. Beckwith (1958). The oxidation of crys- talline cholesterol. Proc. Chem. Soc., 194–195.
- A. L. J. Beckwith (1960). Reactions of alkoxy radicals. I. The reactions of di-tert.-butyl perox- ide with n-butyric acid and ethyl n-butyrate. Aust. J. Chem. 13, 244–255.
- A. L. J. Beckwith (1960). Reactions of alkoxy rad- icals. II. Thermal decomposition of n-octyl nitrite in butyric acid. Aust. J. Chem. 13, 321–323.
- A. L. J. Beckwith (1960). Free-Radical Substi- tution in aromatic compounds. Proc. Roy. Aust. Chem. Inst. 27, 400–404.
- A. L. J. Beckwith and M. J. Thompson (1961).Free-radical phenylationofphenanthrene. J. Chem. Soc., 73–80.
- A. L. J. Beckwith and Low Beng See (1961). Thiyl radicals. Part I. Reactions of anthracene with oxygen and thiols. J. Chem. Soc., 1304–1311.
- A. L. J. Beckwith (1961). Hydroxylation of 5α, 6β-dibromocholestan-3β-ylacetateby chromic acid. J. Chem. Soc., 3162–3164.
- A. L. J. Beckwith and G. W. Evans (1962). Mech- anism of the reactions of peresters catalyzed by copper salts. Proc. Chem. Soc., 63–64.
- A. L. J. Beckwith and G. W. Evans (1962). Reac- tions of alkoxy radicals. Part III. Formation of esters from alkyl nitrites. J. Chem. Soc., 130–137.
- A. L. J. Beckwith (1962). Reaction of anthracene with free radicals derived from 2,2,4- trimethylpentane (isooctane). J. Chem. Soc., 2248–2257.
- A. L. J. Beckwith and R. J. Leydon (1963). The mechanism of the reaction of ferrocene with free radical reagents. Tetrahedron Lett. 4, 385–388.
- A. L. J. Beckwith and L. B. See (1963). Thiyl radi- cals. II. Reactions of meso-substituted anthracene derivatives with oxygen and mercaptoacetic acid. Aust. J. Chem. 16, 845–53.
- D. H. R. Barton and A. L. J. Beckwith (1963). A novel Synthesis of lactones. Proc. Chem. Soc., 335.
- A. L. J. Beck and L. B. See (1964). Thiyl rad- icals. III. Some reactions of anthracene, 1,2- benzanthracene, and 3,4-benzopyrene with thiols and oxygen. Aust. J. Chem. 17, 109–118.
- A. L. J. Beckwith and R. J. Leydon (1964). Free- radical substitution of ferricinium ion: the mech- anism of the arylation of ferrocene. Tetrahedron 20, 791–801.
- A. L. J. Beckwith and R. J. Leydon (1964). Free- radical phenylation of ferricenium ion. J. Am. Chem. Soc. 86, 952–953.
- A. L. J. Beck and Beng See Low (1964). Thiyl radicals. Part IV. Reactions catalyzed by ferrous ion, of polycyclic aromatic hydrocarbons with t-butyl hydroperoxide and mercapto compounds. J. Chem. Soc., 2571–2578.
- B. Acott and A. L. J. Beckwith (1964). Reac- tions of alkoxy radicals. IV. Intramolecular hydrogen-atom transfer in the presence of cupric ion: a novel directive effect. Aust. J. Chem. 17, 1342–1355.
- D. H. R. Barton, A. L. J. Beckwith and Goosen (1965). Photochemical transforma- tions. Part XVI. A novel synthesis of lactones. J. Chem. Soc., 181–190.
- B. Acott and A. L. J. Beckwith (1965). Reaction of lead tetra-acetate with primary amides. Forma- tion of acylamines. Chem. Commun. (London), 161–162.
- A. L. J. Beckwith and J. E. Goodrich (1965). Free-radical rearrangements of N-chloro amides: a synthesis of lactones. Aust. J. Chem. 18, 747– 757.
- A. L. J. Beckwith and J. E. Goodrich (1965). Some oxidation reactions of branched-chain carboxylic acids. Aust. J. Chem. 18, 1023–1033.
- B. Acott, A. L. J. Beckwith, A. Hassanali and J. W. Redmond (1965). Reaction of lead tetra- acetate with primary amides. Formation of alkyl carbamates. Tetrahedron Lett. 6, 4039–4045.
- A. L. J. Beckwith and R. J. Leydon (1966). Co-oxidation of ferrocene and hydrazine deriva- tives. Formation of substituted ferrocenes. Aust. J. Chem. 19, 1381–1390.
- A. L. J. Beckwith and R. J. Leydon (1966). The reaction of ferricinium ion with phenylazotriph- enylmethane. Aust. J. Chem. 19, 1853–1858.
- A. L. J. Beckwith and J. W. Redmond (1966). Reaction of carbethoxynitrene with anthracene, phenanthrene, and pyrene. Aust. J. Chem. 19, 1859–1870.
- A. L. J. Beckwith and J. W. Redmond (1967). Reaction of anthracene with ethoxycarbonyl- nitrene: concentration dependence of prod- uct composition. Chem. Commun. (London), 165–166.
- A. L. J. Beckwith and J. W. Redmond, (1968). Temperature dependence of product composition in reactions of carbethoxynitrene with anthracene and with 2-butene. J. Am. Chem. Soc. 90, 1351–1353.
- A. L. J. Beckwith and R. J. Leydon (1968). The reaction of anthracene with benzoyl radicals. Aust. J. Chem. 21, 817–821.
- B. Acott, A. L. J. Beckwith and A. Hassanali. (1968). Reactions of lead tetraacetate. I. Forma- tion of acylamines from primary carboxamides. Aust. J. Chem. 21, 185–195.
- B. Acott, A. L. J. Beckwith and A. Hassanali (1968). Reactions of lead tetraacetate. II. For- mation of carbamic acid esters from primary carboxamides. Aust. J. Chem. 21, 197–205.
- A. L. J. Beckwith and R. J. Hickman (1968). Reactions of lead tetraacetate. Part III. Forma- tion of pyrimidinediones and related compounds from dicarboxylic acid amides. J. Chem. Soc. C, 2756–2759.
- A. L. J. Beckwith, W. B. Renfrow, A. Renfrow and J. K. Teubner (1968). Transannular rearrangement in 9,10-dihydroanthracene derivatives. Tetrahe- dron Lett. 9, 3463–3464.
- D. L. Struble, A. L. J. Beckwith and G. E. Gream (1968). Cyclization of 4-(1-cyclohexenyl) butyl radical. Tetrahedron Lett.9, 3701–3704.
- A. L. J. Beckwith and R. O. C. Norman (1969). Electron spin resonance studies. Part XX. The generation of organic radicals by the one-electron reduction of aliphatic halogeno- compounds in aqueous solution. J. Chem. Soc. B, 400–403.
- A. L. J. Beckwith and R. O. C. Norman (1969). Electron spin resonance studies. Part XXI. Reac- tions of the phenyl and substituted phenyl rad- icals in aqueous solution. J. Chem. Soc. B, 403–412.
- A. L. J. Beckwith and W. B. Gara (1969). Elec- tron paramagnetic resonance spectral study of intramolecular reactions of aryl radicals. J. Am. Chem. Soc. 91, 5689–5691.
- A. L. J. Beckwith and W. B. Gara (1969). Intramolecular addition and hydrogen atom trans- fer reactions of aryl radicals. J. Am. Chem. Soc. 91, 5691–5692.
- A. L. J. Beckwith (1970). Synthesis of amides, in The Chemistry of Amides (ed. J. Zabicky). New York: Wiley Interscience, pp. 73–186.
- A. L. J. Beckwith (1970). EPR spectral studies of aryl radical reactions. Intra-Science. Chem. Rep. 4, 127–137.
- D.L. Struble, A. L. J. Beckwith and G. E. Gream (1970). Copper-ion catalysed decomposition of bis-5-(1-cyclohexenyl)pentanoyl peroxide. Tetra- hedron Lett. 11, 4795–4798.
- A. L. J. Beckwith (1970). Some aspects of free-radical rearrangement reactions, in Essays on free-radical chemistry (ed. R.O.C. Norman). Chem. Soc., Spec. Publ. No. 24, 239–269.
- A. L. J. Beckwith and G. Phillipou (1971). Stere- oelectronic effects in radical fragmentation: rear- rangement of 3β,5-cyclocholestan-6-yl radical. J. Chem. Soc. D., 658–659.
- A. L. J. Beckwith and P.K. Tindal (1971). Free rad- ical acetoxy group migration; an E.P.R. spectral study. Aust. J. Chem. 24, 2099–2116.
- A. L. J. Beckwith, G. E. Gream and D. L. Struble (1972). Cyclization and cupric-ion oxidation of 4-(cyclohex-1-enyl)butyl radical. Aust. J. Chem. 25, 1081–1105.
- A. L. J. Beckwith (1972). Phosphorous and hypophosphorous acid derived radicals and their reactions. An E.P.R. study. Aust. J. Chem. 25, 1887–1905.
- A. L. J. Beckwith (1973). Free radical structure, reactivity, and rearrangement, in Free radical reactions (ed. W.A. Waters). MTP (Med. Tech. Publ. Co.) Int. Rev. Sci.: Org. Chem., Ser. One 10, 1–47. Butterworth: London, Baltimore.
- A. L. J. Beckwith and C. B. Thomas (1973). Mech- anism of the rearrangement of β-acyloxyalkyl radicals. J. Chem. Soc., Perkin Trans. 2, 861–872.
- A. L. J. Beckwith and G. Phillipou (1973). Cyclization of 5,9-decadienyl and 2-(3-butenyl) cyclohexyl radicals. J. Chem. Soc., Chem. Com- mun., 280–281.
- A. L. J. Beckwith and M. D. Lawton (1973). For- mation and electron spin resonance spectra of cyclic alkoxynitroxide radicals. J. Chem. Soc., Perkin Trans. 2, 2134–2137.
- A. L. J. Beckwith and G. Phillipou (1974). Cop- per salt catalysed reactions of t-butyl perbenzoate with cis- and trans-p-menth-2-ene. Tetrahedron Lett. 15, 79–82.
- A. L. J. Beckwith, I. Blair and G. Phillipou (1974). Preferential cis cyclization of 6-hepten-2-yl and related radicals. Example of orbital symmetry control. J. Am. Chem. Soc. 96, 1613–1614.
- A. L. J. Beckwith, R. T. Cross and G. E. Gream (1974). The mechanismof lead tetraacetate decar- boxylation. I. Tertiary carboxylic acids. Aust. J. Chem. 27, 1673–92.
- A. L. J. Beckwith, R. T. Cross and G. E. Gream (1974). The mechanism of lead tetraacetate decarboxylation. II. 2,3,3-Trimethylbutanoic and adamantane-2-carboxylic acids. Aust. J. Chem. 27, 1693–1710.
- A. L. J. Beckwith and G. Moad (1974). Intramolecular addition in hex-5-enyl, hept-6- enyl, and oct-7-enyl radicals. J. Chem. Soc., Chem. Commun., 472–473.
- A. L. J. Beckwith, I. A. Blair and G. Phillipou (1974). Substituent effects on the cyclization of hex-5-enyl radical. Tetrahedron Lett. 15, 2251– 2254.
- A. L. J. Beckwith, A. K. Ong and D. H. Solomon (1975). Cyclopolymerization. II. Electron spin resonance studies of the free-radical reactions of some diolefins. J. Macromol. Sci., Pure Appl. Chem. A9, 115–124.
- A. L. J. Beckwith, A. K. Ong and D. H. Solomon (1975). Cyclopolymerization. III. Electron spin resonance studies of diallylamines with redox systems. J. Macromol. Sci., Pure Appl. Chem. A9, 125–147.
- A. L. J. Beckwith and W. B. Gara (1975). Intramolecular reactions of ortho-substituted aryl radicals. J. Chem. Soc., Perkin Trans. 2, 593–600.
- A. L. J. Beckwith and W. B. Gara (1975). Mech- anism of cyclization of aryl radicals containing unsaturated ortho-substituents. J. Chem. Soc., Perkin Trans. 2, 795–802.
- A. L. J. Beckwith and G. Moad (1975). Cycliza- tion of 3-allylhex-5-enyl radical: mechanism, and implications concerning the structures of cyclopolymers. J. Chem. Soc., Perkin Trans. 2, 1726–1733.
- A. L. J. Beckwith and G. G. Vickery (1975). Displacement of the hydroxy group from ferro- cenylmethanol by amines. J. Chem. Soc., Perkin Trans. 1, 1818–1821.
- A. L. J. Beckwith and G. Phillipou (1975). GC retention data of C7-C11 hydrocarbons. J. Chro- matog. 120, D6 (Table 961).
- A. L. J. Beckwith and G. Phillipou (1975). GC retention data of p-menthenols. J. Chromatog. 120, D6 (table 962).
- A. L. J. Beckwith and G. Phillipou (1976). Specific β-scission of 3β,5-cyclocholestan-6-yl radical. Aust. J. Chem. 29, 123–31.
- A. L. J. Beckwith and G. Phillipou (1976). E2 reactions of menthyl and neoisomenthyl toluene- p-sulphonates. Aust. J. Chem. 29, 877–882.
- A. L. J. Beckwith, D. G. Hawthorne and D. H. Solomon (1976). Cyclopolymerization. XII. Electron spin resonance studies of the free radical reactions of β-substituted diallylamines. Aust. J. Chem. 29, 995–1003.
- A. L. J. Beckwith and G. Phillipou (1976). Copper-catalyzed reactions of t-butyl perben- zoate with cis- and trans-p-menth-2-ene and other cyclic olefins. Aust. J. Chem. 29, 1277– 1294.
- A. L. J. Beckwith and M. L. Gilpin (1977). The preparation from cyclopentadiene trimer of alco- hols and ketones containing the perhydro- 4,9:5,8- dimethanobenz[f]indene ring system. J. Chem. Soc., Perkin Trans. 1, 19–27.
- A. L. J. Beckwith, C. L. Bodkin and T. Duong (1977). Interaction of ozone with 3,7- dimethyloctyl acetate on solid adsorbents. Chem. Lett., 425–428.
- G. Brunton, K. U. Ingold, B. P. Roberts, L. J. Beckwith and P. J. Krusic (1977). Carbon- 13 and proton hyperfine splittings and their variation with temperature for some alkoxyalkyl radicals. J. Am. Chem. Soc. 99, 3177–3179.
- A. L. J. Beckwith, C. L. Bodkin and T. Duong (1977). Reactions of organic compounds in adsorbed monolayers. I. Ozonation of 3,7- dimethyloctyl acetate. Aust. J. Chem. 30, 2177–2188.
- A. L. J. Beckwith and G. Moad (1977). Aluminium-chloride-promoted reactions of ethyl acrylate with olefins. Aust. J. Chem. 30, 2733– 2739.
- A. L. J. Beckwith (1977). Ring formation and fragmentation in free radicals. Colloq. Int. C. N. R. S. 278, 373–385.
- A. L. J. Beckwith and T. Duong (1978). Regios- elective oxidation of unactivated methylene and methine groups by dry ozonation: similarity to microbiological oxidation. J. Chem. Soc., Chem. Commun., 413–414.
- A. L. J. Beckwith and C. Easton (1978). A stere- oelectronic effect in hydrogen atom abstraction from a substituted cyclohexyl radical. J. Am. Chem. Soc. 100, 2913–2914.
- A. L. J. Beckwith and T. Duong (1979). Regios- elective oxidation of adsorbed alkyl hydrogen succinates by ozone in Freon 11. J. Chem. Soc., Chem. Commun., 690–691.
- A. L. J. Beckwith and T. Lawrence (1979). The effect of non-bonded interactions on the regios- electivity of cyclization of the 5-hexenyl radical. J. Chem. Soc., Perkin Trans. 2, 1535–1539.
- A. L. J. Beckwith and R D. Wagner (1979). For- mation of cyclic peroxides by oxygenation of thiophenol-diene mixtures. J. Am. Chem. Soc. 101, 7099–7100.
- A. L. J. Beckwith and K.U. Ingold (1980) Free- radical rearrangements. In Rearrangements in Ground and Excited States (ed. P. De Mayo), pp. 161–310. New York: Academic Press.
- A. Effio, D. Griller, K. U. Ingold, A. L. J. Beckwith and A.K. Serelis (1980). Allylcarbinyl- cyclopropylcarbinyl rearrangement. J. Am. Chem. Soc. 102, 1734–1736.
- A. L. J. Beckwith and G. Moad (1980). The kinetics and mechanism of ring opening of radicals containing the cyclobutylcarbinyl system. J. Chem. Soc., Perkin Trans. 2, 1083– 1092.
- A. L. J. Beckwith and G. Moad (1980). Ring- opening of some radicals containing the cyclo- propylmethyl system. J. Chem. Soc., Perkin Trans. 2, 1473–1482.
- A. L. J. Beckwith, C. J. Easton and A. K. Serelis (1980). Some guidelines for radical reactions. J. Chem. Soc., Chem. Commun., 482–483.
- A. L. J. Beckwith, T. Lawrence and A. K. Serelis (1980). Stereoselectivity of ring closure of sub- stituted hex-5-enyl radicals. J. Chem. Soc., Chem. Commun., 484–485.
- A. L. J. Beckwith and R. D. Wagner (1980). Regiospecific, stereospecific ring closure of alkenylperoxyl radicals generated by oxygenation of benzenethiol-triene mixtures. J. Chem. Soc., Chem. Commun., 485–486.
- A. L. J. Beckwith and C. J. Easton (1981). Stere- oelectronic effects in hydrogen atom abstraction from substituted 1,3-dioxanes. J. Am. Chem. Soc. 103, 615–619.
- A. L. J. Beckwith and G. F. Meijs (1981). Formation of dihydrobenzofurans by radical cyclization. J. Chem. Soc., Chem. Commun., 136–137.
- A. L. J. Beckwith, G. Phillipou and A. K. Serelis (1981). Formation of some bicyclic sys- tems by radical ring-closure. Tetrahedron Lett. 22, 2811–2814.
- J. E. Baldwin, A. L. J. Beckwith, A. P. Davis, G. Procter and, K. A. Singleton (1981). 5- Isothiazolidinonyl and 5-isoxazolidinonyl radi- cals. Approaches to the biogenetic-type synthesis of β-lactams. Tetrahedron 37, 2181–2189.
- A. L. J. Beckwith (1981). Regio-selectivity and stereo-selectivity in radical reactions. Tetrahe- dron 37, 3073–3100.
- A. L. J. Beckwith and R.D. Wagner (1981). Thiol-oxygen cooxidation reactions of cyclopen- tene, cis- and trans-but-2-ene, norbornene, and norbornadiene. J. Org. Chem. 46, 3638–3645.
- A. L. J. Beckwith and G. F. Meijs (1981). Reac- tions of o-alkenyloxy-arenediazonium fluorobo- rates and related species with nitroxides. J. Chem. Soc., Chem. Commun., 595– 597.
- A. L. J. Beckwith, J. R. Rodgers and R. D. Wagner (1982). Hydroxy sulfoxides derived from nor- bornene: determination of stereochemistry, and synthesis by stereoselective oxidation. Aust. J. Chem. 35, 989–996.
- J. L. Plummer, A. L. J. Beckwith, F. N. Bastin, J. F. Adams, M. J. Cousins and P. Hall (1982). Free radical formation in vivo and hepatotoxicity due to anesthesia with halothane. Anesthesiology 57, 160–166.
- A. L. J. Beckwith and C. J. Easton (1983). Stereo- electronic effects in hydrogen- atom transfer reac- tions of substituted cyclohexyl radicals. J. Chem. Soc., Perkin Trans. 2, 661–668.
- P. J. Barker, A. L. J. Beckwith and Y. Fung (1983). Reversible coupling of a substituted allylic radi- cal with molecular oxygen in a toco reaction of 5-methylhepta-1,3,6-triene. Tetrahedron Lett. 24, 97–100.
- A. L. J. Beckwith, C. J. Easton, T. Lawrence and A. K. Serelis (1983). Reactions of methyl- substituted 5-hexenyl and 4-pentenyl radicals. Aust. J. Chem. 36, 545–556.
- A. L. J. Beckwith, P. H. Eichinger, B. A. Mooney and R. H. Prager (1983). Amine autoxidation in aqueous solution. Aust. J. Chem. 36, 719–739.
- A. L. J. Beckwith and S. H. Goh (1983). Interme- diacy of aryl radicals and arylmetal compounds in reductive dehalogenation of haloarenes with lithium aluminum hydride. J. Chem. Soc., Chem. Commun., 905–906.
- A. L. J. Beckwith and S. H. Goh (1983). Homolytic reductive dehalogenation of aryl and alkyl halides by lithium aluminum hydride. J. Chem. Soc., Chem. Commun., 907.
- A. L. J. Beckwith, D. M. O’Shea and D. H. Roberts (1983). Formation of tetrahydroindans and related systems by methods involving radi- cal ring closure. J. Chem. Soc., Chem. Commun., 1445–1446.
- A. L. J. Beckwith and C. J. Easton (1983). Forma- tion of β-lactams from 3- phenylthiopropionamide derivatives: a possible model for penicillin biosynthesis. Tetrahedron 39, 3995–4001.
- A. L. J. Beckwith, R. Kazlauskas and M. R. Syner- Lyons (1983). β-Fission of 9-decalinoxyl rad- icals: reversible formation of 6-ketocyclodecyl radical. J. Org. Chem. 48, 4718–4722.
- A. L. J. Beckwith and S. W. Westwood (1983). Reactions of cyclohexenyl halides with tributyl- stannane. Stereoelectronic effects on SH 2 reac- tions at halogen. Aust. J. Chem. 36, 2123–2132.
- A. L. J. Beckwith (1984). Carbon-centred rad- icals: fragmentation and rearrangement reac- tions. In Radical Reaction Rates in Liquids (ed. H. Fischer) Landholt-Börnstein Numerical Data and Functional Relationships in Science and Technology, Vol 13, subvolume a, pp. 252–317. Berlin: Springer-Verlag.
- S. Saebo, A. L. J. Beckwith and L. Radom (1984). Mechanism of 1,2-migration in β-(acyloxy)alkyl radicals. J. Am. Chem. Soc. 106, 5119–5122.
- P. J. Barker and A. L. J. Beckwith (1984). E.s.r. identification of alkoxythiocarbonyl radicals as possible intermediates in Barton deoxygenation of alcohols. J. Chem. Soc., Chem. Commun., 683–684.
- A. L. J. Beckwith and C. H. Schiesser (1985). A force-field study of alkenyl radical ring closure. Tetrahedron Lett. 26, 373–376.
- A. L. J. Beckwith and D. R. Boate (1985). Rear- rangement of an o-substituted phenyl radical by 1,7-hydrogen atom migration. J. Chem. Soc., Chem. Commun., 797–798.
- A. L. J. Beckwith and D. R. Boate (1985). Forma- tion of fused bi- and tri-cyclic β-lactams by radi- cal ring closure.Tetrahedron Lett. 26, 1761–1764.
- L. J. Johnston, J. Lusztyk, D. D. M. Wayner, N. Abeywickreyma, A. L. J. Beckwith, J. C. Scaiano and K. U. Ingold (1985). Abso- lute rate constants for reaction of phenyl, 2,2-dimethylvinyl, cyclopropyl, and neopentyl radicals with tri-n-butylstannane. Comparison of the radical trapping abilities of tri-n- butylstannane and -germane. J. Am. Chem. Soc. 107, 4594–4596.
- P. Barker, A. L. J. Beckwith, W. R. Cherry and R. Huie (1985). Characterization of spin adducts obtained with hydrophobic nitrone spin traps. J. Chem. Soc., Perkin Trans. 2, 1147–1150.
- A. L. J. Beckwith, D. H. Roberts, C. H. Schiesser and A. Wallner (1985). Formation of linear triquinanes by serial homolytic cyclization. Tetra- hedron Lett. 26, 3349–3352.
- A. L. J. Beckwith and C. H. Schiesser (1985). Regio- and stereo-selectivity of alkenyl radical ring closure: a theoretical study. Tetrahedron 41, 3925–3941.
- A. L. J. Beckwith and P. E. Pigou (1986). Rela- tive reactivities of various sulfides, selenides and halides towards SH2 attack by tributyltin radicals. Aust. J. Chem. 39, 77–87.
- A. L. J. Beckwith and P. E. Pigou (1986). Forma- tion of lactones via a radical ring closure mecha- nism. J. Chem. Soc., Chem. Commun., 85–86.
- A. L. J. Beckwith and P. E. Pigou (1986). Rel- ative reactivities of various sulfides, selenides, and halides towards SH 2 attack by tributylgermyl radicals. Aust. J. Chem. 39, 1151– 5.
- A. L. J. Beckwith and D. R. Boate (1986). Stere- ochemistry of intramolecular homolytic substitu- tion at the sulphur atom of a chiral sulphoxide. J. Chem. Soc., Chem. Commun., 189–190.
- G. F. Meijs, J. F. Bunnett and A. L. J. Beckwith (1986). Product ratio variation in reactions of o-(3-butenyl)halobenzenes and 6-bromo-1-hexene with alkali metals in ammonia/tert-butyl alcohol solution. Indications of reaction-during-mixing effects. J. Am. Chem. Soc. 108, 4899–4904.
- A. N. Abeywickrema and A. L. J. Beckwith (1986). Homolytic reductive dehalogenation of aryl halides by sodium borohydride. Tetrahedron Lett. 27, 109–112.
- G. F. Meijs and A. L. J. Beckwith (1986). For- mation of functionalized dihydrobenzofurans by radical cyclization. J. Am. Chem. Soc. 108, 5890– 5893.
- A. L. J. Beckwith and D. H. Roberts (1986). Formation of some bi- and tricyclic systems by radical ring closure. J. Am. Chem. Soc. 108, 5893–5901.
- A. N. Abeywickrema, A. L. J. Beckwith (1986). Rate constants for the cyclisation of some aryl radicals bearing unsaturated ortho-substituents. J. Chem. Soc., Chem. Commun., 464–465.
- A. L. J. Beckwith, V. W. Bowry, M. O’Leary, G. Moad, E. Rizzardo and D. H. Solomon (1986). Kinetic data for coupling of primary alkyl radi- cals with a stable nitroxide. J. Chem. Soc., Chem. Commun., 1003–1004.
- A. L. J. Beckwith (1986). Mechanism and appli- cations of free radical cyclization. Rev. Chem. Intermed. 7, 143–154.
- A. L. J. Beckwith and D. M. O’Shea (1986). Kinetics and mechanism of some vinyl radical cyclisations. Tetrahedron Lett. 27, 4525–4528.
- A. L. J. Beckwith, D. M. O’Shea and D. H. Roberts (1986). Novel formation of bis-allylic products by autoxidation of substituted 1,4-cyclohexadienes. J. Am. Chem. Soc. 108, 6408–6409.
- A. N. Abeywickrema, A. L. J. Beckwith (1986). Mechanistic and kinetic studies of the thiodediazoniation reaction. J. Am. Chem. Soc. 108, 8227–8229.
- A. L. J. Beckwith and A. A. Zavitsas (1986). Allylic oxidations by peroxy esters catalyzed by copper salts. The potential for stereoselective syntheses. J. Am. Chem. Soc. 108, 8230–8234.
- A. L. J. Beckwith and S. A. Glover (1987). Determination of the rates of ring-closure of oxygen-containing analogs of hex-5-enyl radical by kinetic electron spin resonance spectroscopy. Aust. J. Chem. 40, 157–173.
- A. L. J. Beckwith and S. A. Glover (1987). An E.S.R. investigation of ethoxy- and trimethyl- silyloxyiminyl radicals. Aust. J. Chem. 40, 701–709.
- A. L. J. Beckwith and G. F. Meijs (1987). Iododediazoniation of arenediazonium salts accompanied by aryl radical ring closure. J. Org. Chem. 52, 1922–1930.
- A. N. Abeywickrema and A. L. J. Beckwith (1987). Mechanistic and kinetic studies on the iododediazoniation reaction. J. Org. Chem. 52, 2568–2571.
- A. N. Abeywickrema, A. L. J. Beckwith and S. Gerba (1987). Consecutive ring closure and neophyl rearrangement of some alkenylaryl rad- icals. J. Org. Chem. 52, 4072–4078.
- A. L. J. Beckwith and S. Brumby (1987). Numer- ical analysis of EPR spectra. 7. The simplex algorithm. J. Magn. Reson. 73, 252–259.
- A. L. J. Beckwith and S. Brumby (1987). Numer- ical analysis of EPR spectra. 8. Relative concen- trations. J. Magn. Reson. 73, 260–267.
- A. L. J. Beckwith and S. Brumby (1987). An elec- tron spin resonance investigation of free radicals with oxygen- and sulfur-containing substituents. J. Chem. Soc., Perkin Trans. 2, 1801–1807.
- A. L. J. Beckwith, D. M. O’Shea, S. Gerba and S. W. Westwood (1987). Cyano or acyl group migration by consecutive homolytic addition and β-fission. J. Chem. Soc., Chem. Commun., 666–667.
- A. L. J. Beckwith (1987). Synthetic Applica- tions and Biosynthetic Significance of Radical Cyclization. Proceedings of the First Princess Chulaborn Science Congress, Natural Products Vol. III, pp. 259–268.
- A. L. J. Beckwith, S. Wang and J. Warkentin (1987). Intramolecular radical additions to the azo group. Fast and indiscriminate 5-exo and 6-endo cyclizations. J. Am. Chem. Soc. 109, 5289–5291.
- A. L. J. Beckwith (1988). Intramolecular Radical Reactions of the Carbonyl Group. Table-Ronde Roussel-Uclaf No. 62 (Paris), p. 25.
- A. L. J. Beckwith, A. G. Davies, I. G. E. Davison, Maccoll and M. H. Mruzek (1988). The mechanisms of the rearrangements of allylic hydroperoxides. J. Chem. Soc., Chem. Commun., 475–476.
- A. L. J. Beckwith, D. M. O’Shea and S. W. Westwood (1988). Rearrangement of suitably constituted aryl, alkyl, or vinyl radicals by acyl or cyano group migration. J. Am. Chem. Soc. 110, 2565–2575.
- A. L. J. Beckwith, V. W. Bowry and G. Moad (1988). Kinetics of the coupling reactions of the nitroxyl radical 1,1,3,3-tetramethylisoindoline-2- oxyl with carbon-centered radicals. J. Org. Chem. 53, 1632–1641.
- Z. Benko, B. Fraser-Reid, P. S. Mariano and L. J. Beckwith (1988). Conjugate addi- tion of methanol to α-enones: photochemistry and stereochemical details. J. Org. Chem. 53, 2066–2072.
- A. L. J. Beckwith and B. P. Hay (1988). Generation of alkoxy radicals from N- alkoxypyridinethiones. J. Am. Chem. Soc. 110, 4415–4416.
- A. L. J. Beckwith and D. R. Boate (1988). Forma- tion of fused tricyclic azetidinones and pyrrolidi- nones by intramolecular SH 2 processes. J. Org. Chem. 53, 4339–4348.
- A. L. J. Beckwith and P. J. Duggan (1988). Dichotomy of mechanism in the rearrangement of β-(acyloxy)alkyl radicals. J. Chem. Soc., Chem. Commun., 1000–1002.
- A. L. J. Beckwith and B. P. Hay (1989). Kinetics of the reversible β-scission of the cyclopentyloxy radical. J. Am. Chem. Soc. 111, 230–234.
- A. L. J. Beckwith and B. P. Hay (1989). Kinet- ics and mechanism of the exo cyclizations of ω-formylalkyl radicals. J. Am. Chem. Soc. 111, 2674–2681.
- A. L. J. Beckwith and V. W. Bowry (1989). Kinetics and regioselectivity of ring opening of substituted cyclopropylmethyl radicals. J. Org. Chem. 54, 2681–2688.
- A. A. Zavitsas and A. L. J. Beckwith (1989). New potential energy function for bond extensions. J. Phys. Chem. 93, 5419–5426.
- B. P. Hay and A. L. J. Beckwith (1989). Synthesis of N-(alkyloxy)pyridine-2(1H)- thiones: alkylations of the ambident nucleophile pyridine-2(1H)-thione N-oxide and attempted isomerizations of 2-(alkylthio)pyridine N-oxide. J. Org. Chem. 54, 4330–4334.
- A. L. J. Beckwith, A. G. Davies, I. G. E. Davison, A. Maccoll and M. H. Mruzek (1989). The mechanisms of the rearrangements of allylic hydroperoxides: 5α-hydroperoxy-3β- hydroxycholest-6-ene and 7α-hydroperoxy-3β- hydroxycholest-5-ene. J. Chem. Soc., Perkin Trans. 2, 815–824.
- A. L. J. Beckwith, B. P. Hay and G. M. Williams (1989). Generation of alkoxyl radicals from O-alkyl benzenesulfenates. J. Chem. Soc., Chem. Commun., 1202–1203.
- A. L. J. Beckwith and S. W. Westwood (1989). The synthesis of indolizidine and quinolizidine ring systems by free-radical cyclization of 4-aza- 6-methoxycarbonyl-5-hexenyl radicals. Tetrahe- dron 45, 5269–5282.
- A. L. J. Beckwith and L. K. Dyall (1990). Oxida- tive cyclization of diamides by phenyliodoso acetate. Aust. J. Chem. 43, 451–461.
- A. L. J. Beckwith and C. L. L. Chai (1990). Diastereoselective radical addition to derivatives of dehydroalanine and of dehydro- lactic acid. J. Chem. Soc., Chem. Commun., 1087–1088.
- A. L. J. Beckwith, V. W. Bowry and C. H. Schiesser (1991). Ring closure of the 6-methylenecyclodecyl radical. Tetrahedron 47, 121–130.
- A. L. J. Beckwith (1991). Organic free radicals. Chem. Aust. 58, 60–63.
- A. L. J. Beckwith and J. Zimmerman (1991). Cyclization of the 3-tert-butylhex-5-enyl radical: a test of transition-state structure. J. Org. Chem. 56, 5791–5796.
- A. L. J. Beckwith and S. M. Palacios (1991). SRN1 reactions of nucleophiles with radical clocks: rate constants for some radical-nucleophile reactions. J. Phys. Org. Chem. 4, 404–412.
- A. L. J. Beckwith, R. Hersperger and J. M. White (1991). Highly diastereoselective addition reac- tions of a radical derived from a β- ethoxycarbonyl sulfoxide. J. Chem. Soc., Chem. Commun., 1151–1152.
- A. L. J. Beckwith, B. J. Maxwell and J. Tsanakt- sidis (1991). The Generation of Aminyl Radicals from Sulfenamides. Aust. J. Chem. 44, 1809– 1812.
- A. C. Willis, A. L. J. Beckwith and M. J. Tozer (1991). trans-3-Benzoyl-2-tert-butyl-4-isobutyl- 1,3-oxazolidin-5-one. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. C47, 2276–2277.
- A. L. J. Beckwith and I. G. E. Davison (1991). Diastereoselective formation of cyclic carbon- ates by cyclization of alkenyloxycarbonyloxy radicals. Tetrahedron Lett. 32, 49–52.
- A. L. J. Beckwith and S. Gerba (1992). The stereochemistry of ring closure of some mono- substituted o-(but-3-enyl)phenyl radicals. Aust. J. Chem. 45, 289–308.
- A. L. J. Beckwith, C. L. L. Chai and A. C. Willis (1992). cis-2-(tert-Butyl)-4-(p- chlorophenylthio)- 3-phenylacetyl-1,3- oxazolidin-5-one. Acta Crys- tallogr., Sect. C: Cryst. Struct. Commun. C48, 593–594.
- A. L. J. Beckwith, R. A. Jackson and R. W. Longmore (1992). The intermediacy of free aryl radicals in the reaction of ortho- alkenyloxybenzenediazonium salts with fer- rocene. Aust. J. Chem. 45, 857–863.
- A. L. J. Beckwith, M. D. Cliff and C. H. Schiesser (1992). Ring closure of 2,2-dimethyloct-7- en-3-yl radical: a system exhibiting unusual solvent dependence. Tetrahedron 48, 4641– 4648.
- A. L. J. Beckwith and K. D. Raner (1992). Stereochemistry of the reversible cyclization of ω-formylalkyl radicals. J. Org. Chem. 57, 4954–4962.
- A. L. J. Beckwith, V. W. Bowry and K. U. Ingold (1992). Kinetics of nitroxide radical trap- ping. 1. Solvent effects. J. Am. Chem. Soc. 114, 4983–4992.
- A. L. J. Beckwith and M. J. Tozer (1992). The iodination and iodocyclization of some alkenyl- malonates. Tetrahedron Lett. 33, 4975–4978.
- A. L. J. Beckwith, C. L. L. Chai and C. Willis (1992). 2-(tert-Butyl)-cis-5-(p- chlorophenylthio)- trans-5-methyl-1,3-dioxolan- 4-one. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. C48, 1362–1364.
- A. L. J. Beckwith and P. J. Duggan (1992). The mechanism of the β-acyloxyalkyl radical rear- rangement: kinetic and 18O labelling studies. J. Chem. Soc., Perkin Trans. 2, 1777–1783.
- A. L. J. Beckwith, S. Brumby and C. L. L. Chai (1992). An EPR study of free radicals derived from 1,3-dioxolan-4-one and related compounds. J. Chem. Soc., Perkin Trans. 2, 2117–2121.
- G. Moad, E. Rizzardo, D. H. Solomon and A. L. J. Beckwith (1992). Absolute rate constants for radical-monomer reactions: thenitroxide method. Polym. Bull. 29, 647–652.
- Y. Chen, S. Brumby, G. Jacobsen, A. L. J. Beck- with and H. A. Polach (1992). A Novel Appli- cation of the ESR Method: Dating of Insular Phosphorites and reef Limestone. Quaternary Science Review 11, 209–217.
- A. L. J. Beckwith (1993). Centenary Lecture. The pursuit of selectivity in radical reactions. Chem. Soc. Rev. 22, 143–151.
- A. L. J. Beckwith and C. L. L. Chai (1993). Some diastereoselective radical reactions of substi- tuted 1,3-dioxolan-4-ones.Tetrahedron 49, 7871– 7882.
- A. L. J. Beckwith, S. P. Joseph and R. T. A. Mayadunne (1993). Highly diastereoselective formation of substituted indolizidines and quino- lizidines by radical cyclization. J. Org. Chem. 58, 4198–4199.
- A. L. J. Beckwith, S. G. Pyne, B. Dikic, C. L. L. Chai, P. A. Gordon, B. W. Skelton, M. J. Tozer and A. H. White (1993). An efficient synthesis of (2S) and (2R)-N-benzoyl-2-t-butyl- 4-methyleneoxazolidin-5-one. Aust. J. Chem. 46, 1425–1430.
- A. L. J. Beckwith and P. J. Duggan (1993). The mechanism of the β-acyloxyalkyl radical rear- rangement. Part 2. β-acyloxytetrahydropyranyl radicals. J. Chem. Soc., Perkin Trans. 2 9, 1673–1679.
- A. L. J. Beckwith and V. W. Bowry (1994). Kinetics of reactions of cyclopropylcarbinyl rad- icals and alkoxycarbonyl radicals containing stabilizing substituents: implications for their use as radical clocks. J. Am. Chem. Soc. 116, 2710–2716.
- A. L. J. Beckwith and S. A. M. Duggan (1994). Kinetics of intramolecular alkyl radical attack on sulfur in disulfides and thioesters. J. Chem. Soc., Perkin Trans. 2, 1509–1518.
- A. L. J. Beckwith and S. Brumby (1994). Carbon-centred radicals: fragmentation and rear- rangement reactions, In Radical Reaction Rates in Liquids (ed. H. Fischer) Landholt-Börnstein Numerical Data and Functional Relationships in Science and Technology, Vol 18, subvolume a, pp. 171–257. Berlin: Springer-Verlag.
- A. L. J. Beckwith and A. A. Zavitsas (1995). Accurate calculations of reactivities and diastere- oselectivities in complex molecules: an AM1 study of 1,3-dioxolan-4-ones and related oxy- gen heterocycles. J. Am. Chem. Soc. 117, 607–614.
- J. R. Axon and A. L. J. Beckwith (1995). Diastere- oselective radical addition to methyleneoxazo- lidinones: an enantioselective route to α-amino acids. J. Chem. Soc., Chem. Commun., 549–550.
- D. Crich, A. L. J. Beckwith, C. Chen, Q. Yao, I. G. E. Davison, R. W. Longmore, C. Anaya de Parrodi, L. Quintero-Cortes and J. Sandoval- Ramirez (1995). Origin of the “β-oxygen effect” in the Barton deoxygenation reaction. J. Am. Chem. Soc. 117, 8757–8768.
- A. L. J. Beckwith and J. M. D. Storey (1995). Tan- dem radical translocation and homolytic aromatic substitution: a convenient and efficient route to oxindoles. J. Chem. Soc., Chem. Commun., 977–978.
- G.Adamson,A. L. J. Beckwith, M. Kaufmannand C. Willis (1995). Highly selective formation and ring fission of some cyclobutaquinolizidi- nones and cyclobutaindolizidinones. J. Chem. Soc., Chem. Commun., 1783–1784.
- A. L. J. Beckwith, S. P. Joseph, R. T. A. Mayadunne and A. C. Willis (1995). 1,3,4,10b- Tetrahydro -4 -phenylpyrido[2,1-a]isoindole-2,6- dione. Acta Crystallogr., Sect. C: Cryst. Struct. Commun. C51, 1438–40.
- A. L. J. Beckwith, S. P. Joseph, R. T. Mayadunne and A. C. Willis (1995). cis-(+-)-1,2,3,6,11,11a-Hexahydro-6-methyl-4H- benzo[b]quinolizin-4-one.Acta Crystallogr., Sect. C: Cryst. Struct. Commun. C51, 2307–2309.
- S. J. Garden, D. V. Avila, A. L. J. Beckwith, V. W. Bowry, K. U. Ingold and J. Lusztyk (1996). Absolute rate constant for the reaction of aryl rad- icals with tri-n-butyltin hydride. J. Org. Chem. 61, 805–809.
- D. Crich, A. L. J. Beckwith, G. F. Filzen and R. W. Longmore (1996). Free radical chemistry of lactones: ring contractions and expansions. J. Am. Chem. Soc. 118, 7422–7423.
- A. L. J. Beckwith and P. J. Duggan (1996). The mechanism of the β-(acyloxy)alkyl radical rear- rangement: substituent and solvent effects. J. Am. Chem. Soc. 118, 12838–12839.
- A. L. J. Beckwith, K. Drok, B. Maillard, M. Degueil-Castaing and A. Philippon (1997). Formation of substituted macrocyclic ethers by radical cyclisation. Chem. Commun., 499–500.
- A. L. J. Beckwith, D. Crich, P. J. Duggan and Q. Yao (1997). Chemistry of β-(acyloxy)alkyl and β-(phosphatoxy)alkyl radicals and related species: radical and radical ionic migrations and fragmentations of carbon-oxygen bonds. Chem. Rev. 97, 3273–3312.
- A. L. J. Beckwith and A. A. Zavitsas (1997). Accurate calculations of reactivities and diastere- oselectivities in complex molecules: an AM1 study of 1,3-dioxolan-4-ones and related oxy- gen heterocycles. [Erratum to publication #202]. J. Am. Chem. Soc. 119, 7171.
- J. J. Brocks, H.-D. Beckhaus, A. L. J. Beckwith and C. Rüchardt (1998). Estimation of bond dis- sociation energies and radical stabilization ener- gies by ESR spectroscopy. J. Org. Chem. 63, 1935–1943.
- A. L. J. Beckwith and P. J. Duggan (1998). The quasi-homo-anomeric interaction in substi- tuted tetrahydropyranyl radicals: structure and kinetics of formation. Tetrahedron 54, 4623– 4632.
- A. L. J. Beckwith and P. J. Duggan (1998). The quasi-homo-anomeric interaction in substituted tetrahydropyranyl radicals: diastereoselectivity. Tetrahedron 54, 6919–6928.
- A. L. J. Beckwith and D. M. Page (1998). For- mation of some oxygen-containing heterocycles by radical cyclization: the stereochemical influ- ence of anomeric effects. J. Org. Chem. 63, 5144–5153.
- A. Philippon, M. Degueil-Castaing, A. L. J. Beck- with and B. Maillard (1998). Formation of macro- cyclic ethers by free radical cyclization: effects of chain length, substituents, and solvents. J. Org. Chem. 63, 6814–6819.
- D. Crich, X. Huang and A. L. J. Beckwith (1999). Free-radical ring contraction of six-, seven-, and eight-membered lactones by a 1,2-shift mech- anism. A kinetic and 17O NMR spectroscopic study. J. Org. Chem. 64, 1762–1764.
- A. L. J. Beckwith and D. M. Page (1999). Stereoselective cyclisation of the 2- allyloxytetrahydropyran-3-yl radical and related species: the influence of anomeric effects. Tetra- hedron 55, 3245–3254.
- A. L. J. Beckwith and J. S. Poole (2002). Factors Affecting the Rates of Addition of Free Rad- icals to Alkenes – Determination of Absolute Rate Coefficients Using the Persistent Aminoxyl Method. J. Am. Chem. Soc. 124, 9489–9497.
- D. J. Henry, A. L. J. Beckwith and L. Radom (2003). Homoanomeric effect in the 1,2- dimethoxyethyl radical. Aust. J. Chem. 56, 429–436.
- A. L. J. Beckwith and R. T. A. Mayadunne (2004). Diastereoselective radical cyclization reactions; the synthesis of O-methylcorytenchirine. ARKIVOC, (x), 80–93.
- G. A. Adamson, A. L. J. Beckwith and C. L. L. Chai (2004). Highly Diastereoselective Radical Reactions of Substituted Methylideneimidazo- lidinones and Related Systems. Aust. J. Chem. 57, 629–633.
- A. L. J. Beckwith, V. W. Bowry, W. R. Bowman, E. Mann, J. Parr and J. M. D. Storey (2004). The mechanism of Bu3SnH-mediated homolytic aro- matic substitution. Angew. Chem., Int. Ed. 43, 95–98.
- A. L. J. Beckwith and K. U. Ingold (2006). Hanns Fischer: radical pioneer. Helv. Chim. Acta 89, 2061–2081.
- A. L. J. Beckwith (2007). Nonconjugated carbon radicals, In Inorganic radicals, metal complexes and nonconjugated carbon-centred radicals (ed. H. Fischer) Landholt-Börnstein Numerical Data and Functional Relationships in Science and Technology, Vol 26, subvolume A, pp. 179–431. Berlin: Springer-Verlag.
- M. L. Coote, C. Y. Lin, A. L. J. Beckwith and A. A. Zavitsas (2010). A comparison of methods for measuring relative radical stabilities of carbon-centred radicals. Phys. Chem. Chem. Phys. 12, 9597–9610.
- A. L. J. Beckwith and C. H. Schiesser (2011). Treasures from the Free Radical Renais- sance Period – miscellaneous hexenyl rad- ical kinetic data. Org. Biomol. Chem. 9, 1736–1743.
- G. Litwinienko, A. L. J. Beckwith and K. U. Ingold (2011). The frequently overlooked impor- tance of solvent in free radical syntheses. Chem. Soc. Rev. 40, 2157–2163.
- A. L. J. Beckwith, L. M. Jackman, R. Ramage and K. G. Watson (1993). Report of a Panel to Review Australian Research Council Fund- ing of Organic Chemistry Research 1987–1991 (Australian Government Publishing Service).
- Beckwith, A. L. J., ‘Preparation of hetero- cyclic compounds’ German Patent 1926475 (23/12/1970).
- Beckwith, A. L. J., Method for producing het- erocyclic acid anhydrides, US Patent 3887550 (3/6/1975) assigned to Sherwin Williams Co.
- Beckwith, A. L. J., Azaisatoic anhydrides, US Patent 3947416 (30/3/1976) assigned to Sherwin- Williams Co.
- Beckwith, A. L. J., method for producing hetero- cyclic acid anhydrides and pyrimidinediones, US Patent 3947442 (30/3/1976) assigned to Sherwin- Williams Co.
About this memoir
Download memoir (PDF)
This memoir was originally published in Historical Records of Australian Science, vol.23, no.1, 2012. It was written by Ian D. Rae, School of Historical and Philosophical Studies, Faculty of Arts, University of Melbourne. iandrae@bigpond.com
Acknowledgements
I am grateful for the assistance I have received from the Beckwith family and from archivists and librarians including Rosanne Walker at the Australian Academy of Science, Helen Bruce at the University of Adelaide, Miriam Congdon at the University of Western Australia, Nicky Hilton of Oxford University Archives at the Bodleian Library, and Don Cook of the Perth Modernian Society. Others who helped me with information were Dr John Jones, Emeritus Fellow of Balliol College, Ron Flack of the Southern Jazz Club, Athel’s former colleagues at Adelaide and Canberra, and research colleagues in Australia (especially Professor Lew Mander and Dr Algi Serelis) and abroad (especially Dr Keith Ingold and Emeritus Professor Alwyn Davies). Dr Serelis kindly provided the structures in Fig. 6. Several members of the Beckwith chemical family read the manuscript in draft and provided helpful comments.






