
Frank Gibson died in Canberra on 11 July 2008. Frank was a highly distinguished research scientist who will be remembered for his pioneering studies in identifying the branch-point compound in the pathway of biosynthesis of a large number of important aromatic compounds followed by a detailed biochemical and genetic analysis of many of the pathways leading to the aromatic amino acids and the so-called aromatic vitamins. Studies on ubiquinone synthesis and function led to an examination of oxidative phosphorylation and the structure and function of the F1 F0-ATPase in the bacterium Escherichia coli. This work resulted in the formulation of a highly innovative model, involving rotating subunits of the F0 segment within the membrane and offering an explanation for the mechanism linking proton flow and ATP synthesis.
Frank William Ernest Gibson was born in Melbourne on 22 July 1923 to John William (‘Bill’) Gibson and Alice Ruby Gibson (née Hancock). He was the eldest of three children, having two younger sisters. He described himself as a third-generation Australian of Irish and Scottish extraction.His father was employed by the Adelaide SteamshipCompany and eventually became a foreman stevedore working on coal ships. Frank had a happy childhood and remembered very supportive parents who shielded the children from the worst effects of the Great Depression. In a typical understatement, he once said that the only hardship that he remembered was eating bread and dripping and having cardboard in his shoes to cover up the holes.
He attended a public primary school and then went to the Collingwood Technical School where it was planned that he should become a draftsman. After two years he decided that drafting was not his thing and at the age of 14 he left school and looked for work. He was fortunate to obtain a position as junior technician in the Bacteriology Department at the University of Melbourne. While he learnt how to wash and plug test-tubes and to make media, he attended evening classes in a Chemistry diploma course that included Science German and Leaving certificate (that is, Year 11) English. After three years he had passed the early stages of his apprenticeship in the Department and graduated to the research laboratory of Dr (later Professor) Syd Rubbo. The Head of Department, Harold Woodruff, and Syd Rubbo both encouraged Frank to consider taking a university course but his qualifications at the time would not have allowed him to gain entrance to Melbourne University. In 1940, the introduction of a new course in Bacteriology at the University of Queensland provided Frank with the opportunity that he needed. Dr (later Professor) D. F. Gray, who was based in the Veterinary School, was to run the course and he needed a technician. Frank was appointed and with David Gray in charge, worked hard on the new course. The University of Queensland had a more helpful attitude towards his qualifications and decided that if he completed chemistry and physics at matriculation level, they would accept his other studies as having provided the necessary requirements for matriculation. Furthermore the University was prepared to pay his fees. After one year of night school he was able to fulfil all the requirements and then began a Science course, again at night school. By the end of 1946 he had completed two years of the three-year Bachelor of Science degree. He had also spent twelve months in the Army before the University successfully recalled him to the Medical School.At the beginning of 1947, he returned to the Bacteriology Department at the University of Melbourne and on the basis of his studies in Queens-land was accepted into the third year of a BSc course, which he completed at the end of 1948. For the next two years he was employed as a Demonstrator (later Temporary Lecturer) and given the opportunity to start some research on the role of acridines as antibacterials.This led to his first publication (1), which may subsequently have been significant in his winning a scholarship to proceed to a DPhil at Oxford.
In 1949 Frank married Margaret Burvill who was working with Syd Rubbo and Adrian Albert on the mode of action of acridines and 8-hydroxyquinoline.The Australian National University had just been established in Canberra and although it was not yet accepting PhD students, it had a generous postgraduate scholarship scheme that enabled successful students to undertake postgraduate studies in Britain. Frank applied for one of these but was initially unsuccessful. However, to his surprise and delight, not long after receiving a letter rejecting his application he received another reversing the first and offering him a scholarship to work for his DPhil at Oxford with D. D. Woods, who was well known for his discoveries in elucidating the mode of action of the sulphonamides. In Oxford, Margaret got a grant to study with Sir Cyril Hinshelwood. For his DPhil, Frank studied the biosynthesis of methionine using
washed-cell suspensions of various mutants of E. coli. Using this approach, he was able to show that serine was the source of the methyl group of methionine (11). Unfortunately this work, apart from a brief abstract, was not published for about seven years, by which time it was generally accepted that serine was the source of the methyl group of methionine. Before leaving Oxford, Frank received two offers of academic positions. One was a fellowship in the Microbiology School at theAustralian National University and the other was a Senior Lectureship in the Bacteriology Department at the University of Melbourne where S. D. Rubbo was now Head. Frank chose Melbourne and took up his position in 1953.
As a Senior Lecturer Frank taught Bacterial Physiology to third-year students and General Microbiology to second-year students. He was an excellent teacher and was very popular with students. He also established a research laboratory, applying the methodology that he had used at Oxford, namely using washed-cell suspensions of mutant strains of Aerobacter aerogenes and E. coli bacteria, to further elucidate reactions and intermediates in the pathway of biosynthesis of the aromatic amino acids.
The equipment was fairly primitive in those days. The laboratory featured a Unicam manual spectrophotometer (to be replaced later by the Carey automatic machine), a sonicator and a Hughes press for breaking cells, two centrifuges (down the hall), a rotary evaporator that worked off the suction provided by the water taps and that filled with water every time the pressure fluctuated, and several glass battery jars that Frank had scrounged from somewhere and that were used for paper chromatography. Even though several highly toxic solvents such as benzene, pyridine and toluene were used in the chromatography, when the run was finished the paper chromatograms were removed from the jars and attached to a piece of string strung across the room in order to dry. That the inhabitants of the laboratory appear to have survived these noxious fumes without any adverse effects is remarkable.
Early studies carried out with Colin Doy resulted in the identification of a new compound accumulated by certain tryptophan auxotrophs, namely 1-(o-carboxyphenylamino-1-deoxyribulose). Subsequent work showed this to be a dephosphorylated form of the true intermediate, now known as CDRP (10).
In 1959 Frank received a Carnegie Foundation of NewYork travel grant that enabled him and Margaret to travel to the USA where they worked with Charley Yanofsky at Stanford University. Frank also attended Van Niel’s famous summer course in General Microbiology held at the Johns Hopkins Marine Station each year. This experience greatly inspired his teaching of General Microbiology on his return to Melbourne. The work with Yanofsky involved a study of cell-free extracts of E. coli to establish whether one or more enzymes were involved in the conversion of CDRP to indoleglycerol phosphate. Frank achieved a partial purification of indoleglycerol phosphate synthetase and showed that a single protein was involved in the reaction (12).
On his return to Australia after his study leave, Frank was promoted to Reader in Chemical Microbiology and his laboratory switched from whole-cell suspensions to cell-free extracts and also focused on the one remaining unsolved problem of the pathway, namely the position and nature of the hypothetical branch-point compound. All the work leading to the discovery of this intermediate was carried out in the University’s old Bacteriology School.
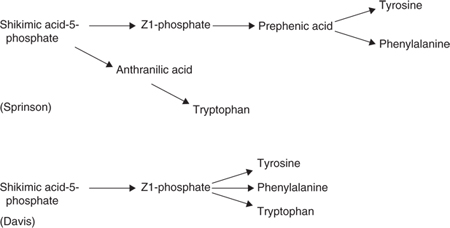
Other workers had shown that the biosynthesis of the aromatic amino acids proceeded via a set of common reactions (the common pathway) with three terminal pathways leading to the amino acids tryptophan, phenylalanine and tyrosine. A compound referred to as Z1-phosphate (3-enolpyruvylshikimate-5-phosphate) and formed from shikimate-5-phosphate was the last intermediate to be identified in the common pathway (see Fig. 1).
One proposal current at the time was that the tryptophan pathway diverged at shikimate-5-phosphate and that the phenylalanine and tyrosine pathways diverged at Z1-phosphate (Sprinson 1960). An alternative model suggested by Davis was that all three pathways diverged from the same compound but whether it was Z1-phosphate or some unknown compound beyond this was not known (see Fig. 1 and (18)).
Doy and Gibson investigated these models with one final washed-cell suspension experiment in which cells of a tryptophan auxotroph blocked after anthranilic acid were grown in a nitrogen-rich medium and then transferred to a nitrogen-free medium. After incubation the supernatants were examined for accumulated products. It was postulated that in the nitrogen-free medium the intermediate directly preceding anthranilic acid might accumulate. The experiment revealed that under these conditions three major aromatic compounds accumulated, 4-hydroxy benzaldehyde, phenylpyruvic acid and 4hydroxyphenylpyruvic acid. As the two phenylpyruvic acids were the first known intermediates in the terminal pathways to phenylalanine and tyrosine it was argued, as it turns out correctly, that when the tryptophan pathway was shut off because of the absence of a nitrogen source, the precursor of anthranilic acid was directed along the terminal pathways to phenylalanine and tyrosine (13). Although the hypothetical compound was not identified, the results offered support for the Davis model and also provided some direction for the next critical experiment.
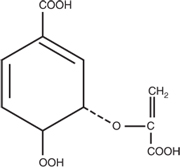
Before taking steps to block the conversion of the hypothetical branch-point compound to the phenylpyruvic acids, experiments were undertaken to establish conditions under which cell-free extracts could convert shikimic acid (a readily available substrate) into anthranilic acid, phenylpyruvic and 4-hydroxyphenylpyruvic acid. During these experiments phosphoenol pyruvic acid, which is required for the formation of Z1-phosphate, was shown to stimulate the formation of anthranilic acid as well as the phenylpyruvic acids, adding further support for the Davis model (23). In a further refinement of these studies, by using cell-free extracts from mutants blocked in successive reactions in the common pathway, it was possible to separate the overall conversion into two discrete steps. In the first, using extracts of A. aerogenes A170–44, which was blocked in the reaction immediately after Z1-phosphate, shikimic acid could be converted to a compound with all the characteristics of Z1-phosphate. In the second, using extracts from A. aerogenes poly 3, which was blocked between shikimate-5-phosphate and Z1-phosphate, this compound could be converted to anthranilic acid and to a lesser extent to phenyl pyruvic and 4-hydroxyphenyl pyruvic acid. This second reaction required DPNH and was postulated to involve the production of the hypothetical branch-point compound (18). Starting with a tryptophan auxotroph of A. aerogenes unable to convert anthranilic acid to tryptophan, a strategy was developed to block reactions converting the hypothetical branchpoint compound to phenylpyruvic and 4-hydroxyphenylpyruvic acid.This involved irradiating the tryptophan auxotroph and isolating a mutant, which now required both tryptophan and tyrosine for growth. This strain was further irradiated and a second strain was isolated, which now required tryptophan, phenylalanine and tyrosine for growth. The observation that this mutant was still able to accumulate anthranilic acid confirmed that the common pathway was still intact and distinguished it from several other mutants that had acquired a requirement for phenylalanine because of a second block in the common pathway. This triple mutant was called 62–1. Cell-free extracts of this strain prepared from cells that had been grown in limiting tryptophan could readily convert shikimic acid to anthranilic acid. When glutamine was omitted from the reaction mixture, to their great excitement a new compound was formed. This compound, which appeared to be the hypothetical branch-point compound formerly called compound X, could be extracted by ethyl acetate or ether after acidification and used as a substrate with extracts ofA170–44 that were able to convert it in the presence of glutamine to anthranilic acid, and in the presence of an oxidized form of diphosphopyridine nucleotide (DPN+) to phenyl pyruvic and 4-hydroxyphenyl pyruvic acid. On more prolonged incubation, extracts of 62–1 were also able to convert shikimic acid to 4-hydroxybenzoic acid (17, 26, 28).After consulting his father-in-law,Archdeacon W. Burvill, who was an accomplished Greek scholar, Frank proposed the name chorismic acid (meaning separation) for the elusive compound X (141).
Attempts to isolate quantities of this compound in a pure state involved what Frank later described as several esoteric and potentially hazardous purification techniques such as chromatography in ether on columns of sucrose.As he stated, these were carried out under conditions that would give a present-day safety officer nightmares. In the end chorismic acid was shown to be less labile than had originally been feared and a relatively simple procedure was developed using ion exchange columns in the cold and subsequent precipitation in methanol as a barium salt (26).
The structure that had been proposed for chorismic acid was confirmed and the stereochemistry defined when Lloyd Jackman, a new Professor of Chemistry at the University of Melbourne, ran a nuclear magnetic resonance (NMR) spectrum on a pure sample of barium chorismate provided by Frank (22). As had been predicted, it was shown to be a hexadiene (3-enolpyruvic ether of trans-3,4-dihydroxycyclohexa-1,5-diene carboxylic acid) (see Fig. 2).
The availability of pure chorismic acid created a great opportunity to study the many divergent pathways that used this compound as a starting point (see Fig. 3).
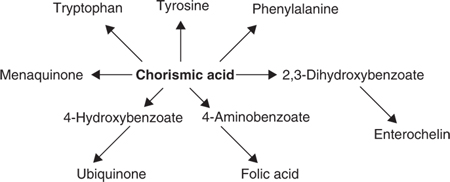
The next ten years or so were extremely productive as Frank and his graduate students and later post-docs pursued many of the pathways with vigour. His first wife Margaret had made significant contributions to much of the work leading to the successful identification and isolation of chorismic acid. Unfortunately, illness prevented her from participating in the next phase of the work. This work was started in a laboratory in the Chemistry Department at the University of Melbourne where Frank’s group was temporarily housed while the new building for the Department of Microbiology was being erected. In 1965 the new building was finished and Frank was promoted to Professor of Chemical Microbiology. During the next two years work began in earnest on the phenylalanine and tyrosine pathways, the pathways for ubiquinone and menaquinone, p-aminobenzoic acid and tryptophan. At this stage he also made the switch to E. coli K-12 and genetic analysis became an important part of his future research. In 1967 Frank was offered and accepted the Chair in Biochemistry at the Australian National University and moved there with many members of his group. The further exploration of these pathways and the extensive investigation of ATP synthase and oxidative phosphorylation all took place at the ANU.
It had been known that an early precursor in both the phenylalanine and tyrosine pathwayswas the compound prephenic acid and its formation from chorismic acid had already been demonstrated. Cotton and Gibson investigated the enzymes involved in its formation. Chromatgraphy of cell-free extracts on DEAE cellulose revealed that there were two discrete and separable proteins with enzymic activity (called chorismate mutase) converting chorismate to prephenate. Of considerable interest was the finding that associated with one of these peaks of mutase activity was activity for the second reaction of the phenylalanine pathway (prephenate dehydratase), which converted prephenate into phenylpyruvate, and associated with the other peak of mutase activity was the second reaction of the tyrosine pathway (prephenate dehydrogenase), which converted prephenate into 4-hydroxyphenylpyruvate (29). Subsequent work on the purified enzymes, some of which was carried out in Gibson’s laboratory, confirmed that both activities in each case were carried on a single protein (65).
The bacterium E. coli contains two major quinones, ubiquinone and menaquinone or vitamin K2. In an early paper Cox and Gibson, having established appropriate methods for the extraction and identification of the quinones, used radio-labelled shikimate to demonstrate that both ubiquinone and menaquinone were products of the shikimic acid pathway. In the same experiments, by using excess unlabelled 4-hydroxybenzoic acid, they showed that this compound was an intermediate in the synthesis of ubiquinone but not in the synthesis of menaquinone (24, 30). Subsequently, by using cell-free extracts of a wild-type strain grown in the presence of the three aromatic amino acids, they were able to demonstrate the conversion of radio-labelled chorismic acid into both ubiquinone and menaquinone. As was reported later, they had also observed that certain multiple aromatic auxotrophs with a complete block in the common pathway could grow on a glucose mineral salts medium supplemented with the three aromatic amino acids, 2,3-dihydroxybenzoic acid and p-aminobenzoate. Under these conditions the cells failed to synthesise either ubiquinone or menaquinone (32).
At about this time, other workers (Daves et al. 1966; Friis et al. 1966) investigated the ubiquinone pathway in Rhodospirillum rubrum by using radio-labelled 4hydroxybenzoate as a growth supplement, extracting large quantities of cells and identifying several radio-labelled polyisoprenoid compounds. On the basis of these results they proposed a hypothetical pathway for ubiquinone biosynthesis. Although the pathway was for the most part subsequently confirmed, the general approach suffered certain limitations. In particular, extremely small amounts of some intermediates were obtained, making their detailed chemical analysis difficult. Other proposed intermediates were not detected and examination of the enzymic reactions was not possible.
Gibson believed that, once again, the use of specific mutants blocked in the biosynthetic pathway should enable a ready and detailed identification of intermediates in the pathway. He chose E. coli K-12 as the starting strain so that a genetic analysis of any mutant strains could be readily carried out.
Because of its fermentative ability, E. coli did not require ubiquinone for growth on glucose, making the isolation of ubiquinone mutants difficult. To overcome this problem, non-fermentable substrates such as succinate or malate were substituted for glucose and under these conditions cells unable to make ubiquinone failed to grow (32). Using nitrosoguanidine as a mutagen and a system of delayed enrichment on solid agar, ∼100 mutants were isolated that could grow on glucose but not on malate. After purification each one of these was grown in 2-L batches, the cells were extracted and the extracts run on chromatograms to detect the presence or absence of ubiquinone. Of the 100 mutants tested, two were found to be unable to synthesise ubiquinone (40, 44). The accompanying genetic analysis proved to be important when the first mutant strain to be isolated was shown to possess four separate mutations, each of which affected growth on malate and two of which involved lesions in the ubiquinone pathway. Genetic techniques were used to establish mutants with a single mutation affecting ubiquinone synthesis and these were then subjected to a detailed analysis. Genetic crosses with Hfr strains followed by transductions were used to locate the mutated genes on the E. coli chromosome and large-scale cultivation of the mutants produced sufficient quantities of the intermediate before the blocked reaction to allow a detailed spectral analysis with NMR, mass spectrometry and infrared spectroscopy. At this stage Cox and Gibson transferred their major interest to oxidative phosphorylation and ATP synthase and Ian Young took a major responsibility for the isolation and characterization of mutants blocked in the remaining five reactions and for the identification of the intermediates formed by these mutants.The overall results of these studies are summarized in Frank Gibson’s special lecture to a joint meeting of the Biochemical Society and the Chemical Society in London in 1972 (82), and in a paper by Young, Stroobant, McDonald and Gibson in 1973 (84).
Early studies with washed-cell suspensions of tryptophan and other aromatic auxotrophs of A. aerogenes had identified 2,3-dihydroxybenzoic acid as a product recoverable from the supernatants, even though its function at that time was unknown (10). Ito and Nielands (1958) had reported that iron-starved cultures of Bacillus subtilis accumulated 2,3dihydroxybenzoylglycine. In 1966 other workers showed that a methionine B12 auxotroph of E. coli accumulated 2,3dihydroxybenzoylserine when grown under conditions in which iron was limiting (Brot et al. 1966). The relationship of these compounds to the chorismic acid pathway was confirmed when Cox and Gibson showed that 2,3-dihydroxybenzoic acid was an essential growth factor for certain multiple aromatic auxotrophs growing on media supplemented with tryptophan, phenylalanine, tyrosine, p-aminobenzoate, p-hydroxybenzoate and 3,4-dihydroxybenzaldehyde. The latter two compounds required for ubiquinone and vitamin K2 biosynthesis were not required for growth on glucose and in their absence growth was still obtained in media supplemented with 2,3-dihydroxybenzoic acid. Under these conditions neither vitamin K nor ubiquinone were made, indicating that 2,3dihydroxybenzoic acid was not a precursor of these quinones. The requirement for 2,3dihydroxybenzoic acid can be replaced by shikimate in those mutants with blocks in the pathway before shikimic acid (32). Cell-free extracts of A. aerogenes (62–1) and of E. coli were shown to convert chorismic acid into 2,3-dihydroxybenzoic acid. Furthermore it was shown that in the absence of DPN and Mg2+ , chorismate was converted to an unknown compound that was then converted to 2,3dihydroxybenzoate when these co-factors were added (36). Further investigation of the synthesis of 2,3-dihydroxybenzoic acid in A. aerogenes was undertaken by Ian Young who with Frank and several graduate students, isolated and identified two intermediates between chorismate and 2,3-dihydroxybenzoate and showed that the synthesis of the enzymes that produced these compounds was repressed by iron (37, 47, 48). The genes encoding the enzymes for the synthesis of 2,3dihydroxybenzoate from chorismate were mapped in E. coli (70), as were the genes for the enzymes involved in the conversion of 2,3-dihydroxybenzoate to the final product, enterochelin (67). The functional iron chelator was shown to be a trimer of 2,3dihydroxybenzoylserine and was named enterochelin (46, 60).At the same time independent studies of S. typhimurium identified a similar compound, which was named enterobactin (Pollack and Nielands 1970). Both terms are still used although outside Australia, the term enterobactin is more commonly encountered.
p-aminobenzoic acid is a precursor of dihydrofolate and was shown in early experiments to be formed from chorismic acid (27). In E. coli, mutants unable to synthesise p-aminobenzoic acid were isolated and two structural genes pabA and pabB were identified. A study of cell-free extracts of these mutants by Huang and Gibson showed that at least two reactions were involved in the conversion of chorismate to p-aminobenzoate (57). No further studies were carried out in Gibson’s laboratory on this pathway.
In the early 1970s Frank and Graeme Cox turned their attention to the study of oxidative phosphorylation, a process in which the conversion of ADP to ATP is linked to the passage of electrons from oxidizable substrates to oxygen. The enzyme complex involved in these reactions, originally termed F1F0-ATPase and now known as ATP synthase had been extensively studied in mitochondria. Factor 1 (F1) was a soluble ATPase isolated from the membranes and F0 was a factor that rendered the ATPase activity of the F1 sensitive to the antibiotic Oligomycin.
Frank was convinced that the opportunities that had been offered by the study of bacterial mutants in the elaboration of biosynthetic pathways would also apply to the problem of a complex like ATP synthase. During the isolation of the various mutants blocked in the synthesis of ubiquinone, several mutants had been isolated, which, although still able to synthesise ubiquinone, were unable to grow on substrates such as succinate or malate. It was argued that, amongst such mutants, there should be some affected in the process of oxidative phosphorylation. In 1971 Butlin, Cox and Gibson described two mutants that were able to grow on glucose but not on succinate or lactate (62).They had normal lactate oxidase and NADH oxidase activities but assays on membrane preparations showed that they lacked ATPase activity and measurement of P/O ratios showed that they were uncoupled with regard to oxidative phosphorylation and electron transport. A further interesting and useful observation was that when grown aerobically on limiting glucose, these mutants had a reduced growth yield. This phenotype was subsequently used to aid in the identification of other mutations affecting this complex. The mutations were mapped by conjugation and transduction to min 73.5 on the then E. coli chromosome and the gene was termed uncA (for uncoupled).
On the basis of their phenotypes, these mutants were assumed to be altered in the F1 component of ATP synthase. This discovery, which was made at a time when bacteria such as E. coli were largely discounted as having any great relevance to the extensive studies of mitochondrial ATP synthase, provided Frank with an opportunity to express his conviction of the potential for these bacterial studies. In this paper he stated: ‘The use of bacteria with their simpler cellular organization than eukaryotic cells, and of E. coli in particular, with its amenity to genetic manipulation seems a promising experimental system for a combined genetic and biochemical approach to the problem of coupling of phosphorylation to electron transport’.
Further examination of the mutant strain allowed them to show that after washing the membranes with a low-ionic-strength buffer ATP-dependent transhydrogenase, activity could be reconstituted by adding purified Mg, Ca ATPase to the washed membranes (81). In 1973 Butlin, Cox and Gibson reported the characterization of a second mutant unable to couple electron transport to oxidative phosphorylation (79). This mutant had wild-type activity for ATPase. It gave a low growth yield aerobically on limiting glucose, as did the uncA mutant, but unlike the uncA mutant it showed no impairment in its ability to grow anaerobically on glucose. The mutation mapped to the same general location as the uncA mutation and it was given the designation uncB. In a subsequent paper Cox, Gibson and McCann carried out a series of reconstitution experiments using washed membranes and soluble fractions from the uncA and the uncB mutant. The only effective combination was the membrane fraction from the uncA mutant with the low-ionic-strength wash from the uncB strain (86). Confirmation that these mutations affected different components of ATP synthase highlighted the need for additional genetic tests other than mapping to identify individual genes. Since this work was carried out well in advance of the gene cloning and DNA sequencing techniques that would later greatly simplify such studies, a system of complementation was developed to distinguish mutations affecting different genes. By using an F’ strain in which the F-genote carried a small deletion including ilv and unc, they created a system that allowed the ready introduction of mutant unc alleles to the F-genote, which could then be easily transferred to other mutants for complementation studies (92).
Using this system, seven of the eight genes encoding the polypeptides of ATP synthase were identified (92, 103, 110). The localized clustering of the various unc genes suggested the possibility that they may be organized into a single transcription unit. In order to test this hypothesis, bacteriophage Mu was used to introduce a series of polar mutations within the unc cluster (95). Complementation tests between these mutants and the known mutant alleles then allowed a clear formulation of gene order (uncBEADC). As more mutants were identified additional genes were included, uncG and uncF. The order uncB(EF)A(DG)C was proposed. The Mu-induced mutants available did not allow the ordering of the uncE and uncF or the uncG and uncD genes. Later cloning of the unc genes revealed the order uncAGD, and the subsequent DNA sequencing of the operon established that the order of the genes encoding the F0 ATPase subunits was uncBEF (Gay and Walker 1981a, 1981b; Sarate et al. 1981). The identification of the last gene of the operon, uncH, which encodes the δ subunit, was reported in (Humbert et al. 1983).
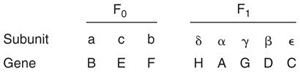
Detailed biochemical studies of the various unc mutants were carried out to identify the particular polypeptides specified by each of the genes. Two-dimensional gel electrophoresis of the polypeptide composition of p-aminobenzamidine-washed membranes from an uncD mutant identified an altered β subunit with a wild-type molecular weight but a different isoelectric point (96). The genes uncB, uncE and uncF appeared to encode the polypeptide components of the F0 membrane-associated part of ATP synthase. Membrane preparations from strains carrying mutations in the uncE, uncBor uncF gene were also examined by two-dimensional gel electrophoresis. Whereas membranes from the uncE and uncB mutants contained an apparently normal 18 000-molecular weight polypeptide, this was missing in the uncF strains. In vitro transcription translation studies indicated that uncB probably encoded the 24 000-molecular component. The 8,400molecular weight polypeptide would then be encoded by uncE (110). The gene polypeptide relationships for both the F0 and F1 components of the ATP synthase are shown in Fig. 4.
The observation that the genes encoding the polypeptides of the F0 component (BEF) were the first to be transcribed in the unc operon gave rise to the idea that perhaps the F0 complex was assembled first. That this was not the case became apparent when a mutant was isolated with unusual characteristics. Complementation analyses showed that although it expressed wild-type functions for the products of uncBEF and G, it was unable to complement uncD and uncC mutants, indicating that it probably had a polarity mutation affecting uncD. Unexpectedly, membranes isolated from this strain were impermeable to protons. Examination of the membrane proteins by two-dimensional gel electrophoresis showed that not only were F1 subunits missing but the Mr 18 000 subunit of the F0 ATPase (the product of the uncF gene) was also missing. By using a series of Mu-induced polarity mutants, it was then shown that a functional uncD product (β subunit) was required for insertion of the Mr 18 000 F0 subunit into the membrane. On the basis of these results a hypothetical scheme was proposed for the assembly of F1F0-ATPase. A perceived advantage of the new scheme was that the attachment of β and α subunits before the proton pore is complete could prevent the free and uncontrolled flow of protons (109, 111). Further studies on several mutants altered in the uncE gene provided supportive evidence to the model in which the small subunit c protein inserts into the membrane as a helical hairpin (113, 115). Complementation between pairs of uncF alleles confirmed a role for subunit b in different stages of the assembly of the F1F0-ATPase and showed that it was present as a dimer (122).
A detailed account of all of this work on ATP synthase is to be found in Frank’s Leeuwenhoek Lecture to the Royal Society of London in 1981 (111).
After the complete DNA sequence of the unc operon (also called atp) became available (Gay and Walker 1981a, 1981b; Saraste et al. 1981), the primary amino acid sequence for each of the subunits was also revealed.
In the mid-to-late 1980s, Gibson’s laboratory published several papers that proposed and then refined an ingenious and novel hypothesis that explained the link between the passage of protons through F0 and the synthesis of ATP in F1 (118, 123, 126–128). The essential feature of the model was that the passage of protons through F0 drove the rotation of one subunit relative to another. In the initial model it was proposed that the b subunit rotated relative to a ring of nine c subunits as a consequence of sequential interactions between the positively charged Lys-23 in the b subunit and successive c subunit Asp-61 residues. This rotation was proposed to drive rotational movements in F1 in which conformational changes associated with the three alternating catalytic sites of F1 ATPase resulted in ATP synthesis. When site-directed mutagenesis showed that Lys-23 residue was not essential for oxidative phosphorylation, the topology of subunit a was re-examined in several species and a new topological model with five membrane spans was proposed and possible interactions between subunits a and c were explored. Span 4 of subunit a had characteristics of an amphipathic helix (123). Mutagenesis showed that Arg-210 of this span was critical for function and was likely to be the positively charged residue with which Asp-61 interacted. Results of further mutagenesis suggested a proton pore through F0 involving His-245, Glu-219 and Arg-210 of spans 5 and 4 of subunit a and Asp-61 of span 2 of the c subunit (123, 126, 127). The underlying logic in the formulation of this model is contained in the following quotation from one of the papers:
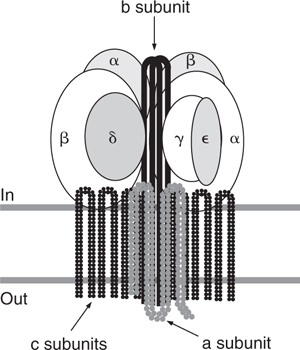
It is of particular interest that in the F0 the proton pore is shared between two subunits of different stoichiometries. The a-subunit is present in F1 F0ATPase as one copy, whereas the c-subunit is present in from six to ten copies. It is likely that all c-subunits are functionally important, which would require the amino acids in the proton pore of the a-subunit to interact with the Asp-61 of each c-subunit. This is a fundamental feature of a rotational catalysis model proposed for ATP synthesis by the F0 F1ATPase.
The model that they proposed is shown in Fig. 5.
Although there have been important modifications, many years later the prescience of this model is apparent.
Whether you measure it from the Collingwood Technical School to the Royal Society of London, or from examining washed-cell suspensions of a bacterium to elucidating the gene–enzyme relationships and the organization of a complex enzyme such as ATP synthase, Frank Gibson’s life was an extraordinary journey. His research, driven by an enquiring mind, was original and pioneering. His discoveries dating from the identification of chorismic acid were pivotal to the development of new pathways of discovery. He never hesitated to pose difficult questions and was not influenced by prevailing norms that directed work into acceptable channels. In the early days Frank said that he subscribed to the view that one experiment is worth a thousand expert opinions. He encouraged his students to get into the laboratory and test their hypotheses. During his lifetime he trained many young aspiring graduate students. All of these, many of whom have proceeded to distinguished positions in science, speak with great affection and respect of his role as a mentor and friend. His enthusiasm for the work and the integrity and self-deprecating honesty that he always applied to research achievements served as a model that was difficult not to take up. With a relatively small group and modest funding he achieved an international reputation for excellence that brought credit to himself and to Australian science. After his retirement in 1988 he was appointed Visiting Fellow in the Membrane Biochemistry Group at the Australian National University and for many years was busy setting up systems for the molecular biological work and the computer modelling of membrane proteins.
In 1959 Frank was awarded a Carnegie Foundation Travel Grant. In 1963 he received the David Syme Research Prize of the University of Melbourne. In 1968 he was selected to give the first Lemberg Lecture to the annual meeting of the Australian Biochemical Society. In 1971 he was elected as a Fellow of the Australian Academy of Science and in 1976 elected to the Fellowship of the Royal Society of London. He gave the S. D. Rubbo Memorial Oration in 1975 and was invited to give the Leeuwenhook Lecture to the Royal Society in London, and in Manchester and Durham in 1981, the Hopkins Memorial Lecture in London in 1982 and the Burnet Lecture in Canberra in 1991.TheAustralian Biochemical Society, the Australian Society of Microbiology and University House at the Australian National University all made him an Honorary Life Member. During his lifetime he was also an invited speaker at several international conferences. Apart from three years as Director and Howard Florey Professor of Medical Research in the John Curtin School of Medical Research at the Australian National University between 1977 and 1980, he was from 1967 to 1988 Professor and Head of the Division of Biochemical Sciences in the John Curtin School. In 1989, he was made an Emeritus Professor and a University Fellow at ANU. As a Visiting Fellow in the Membrane Biochemistry Group he continued his contributions to research in the new area of computer modelling of membrane proteins. In January 2004 he was appointed a Member of the Order of Australia.
At various times Frank was a member of the editorial boards of Biochimica et Biophysica Acta, the Biochemical Journal and the Journal of Bioenergetics and Biomembranes. He was a member of several advisory committees including the Medical Research Advisory Committee of the National Health and Medical Research Council, the Clive and Vera Ramaciotti Foundation, the Sydney Committee of the Ludwig Institute for Cancer Research, the Recombinant DNA Monitoring Committee and the Scientific Advisory Committee of the Centenary Institute of Cancer Medicine and Cell Biology. He was a member of the University House Governing Body (1987– 1992) and a member of the Council of the Australian Academy of Science (1987– 1989).
Outside the laboratory Frank exhibited a great enthusiasm for life, a willingness to take on all sorts of challenges—which he met with a quiet determination—a readiness to question anything that appeared false or overblown, and a quiet friendly persona that endeared him to many. He was also a highly competitive individual and was always very active in a variety of physical activities—in his early years in Queensland, climbing mountains and surfing; back in Victoria, cross-country skiing and scuba diving followed by squash and tennis; and later, in Canberra, swimming in the waters at Guerilla Bay in all seasons and continuing to ski at every opportunity. He never let what he would regard as minor setbacks interfere with his plans. After suffering a major shoulder injury while skiing, he arrived at the tennis court the next day with one arm tightly held in a sling, as ready as always for the morning battle. He maintained an active interest in political events, which he viewed with a well honed cynicism. He was very attached to his children, to Frances and Ruth from his first marriage and to Mark from his second. He followed their activities with great interest and a certain amount of pride. He was greatly saddened when Ruth died of breast cancer and maintained an active involvement with the grandchildren. He and Robin Rollason were married in 1980 and in 1982 they travelled to the UK and stayed in Oxford where Frank was appointed for a year as Newton Abrahams Visiting Professor. Back in Australia, they built a holiday home at Guerilla Bay where close friends Lloyd and Margaret Evans had a house. Many of Frank’s friends and associates spent wonderfully enjoyable weekends with him and Robin, walking, swimming and playing tennis.
This memoir was originally published in Historical Records of Australian Science, vol.21, no.1, 2010. A similar memoir will be published in Biographical Memoirs of Fellows of the Royal Society of London, vol.56, 2010. It was written by:
We should like to thank members of the family for reading the manuscript and Nancy Millis and Ian Young for helpful comments. We should also mention an excellent autobiography written by Frank, published in Comprehensive Biochemistry (139).
© 2026 Australian Academy of Science