Robert Henry Symons 1934–2006

Written by George E. Rogers and William H. Elliott.

- Introduction
- Early Years and Education
- Postdoctoral and Early Academic Career
- The Adelaide Years
- Early Research
- The Preparation of 32P-Labelled Nucleotides
- Plant Viruses and Viroids: Structure, Function and Replication
- Commercialization of Research and Advisory Roles
- External Scientific Contributions
- Later Years at the Waite Agricultural Research Institute: the Wine Industry and Grape Diseases
- About this memoir
Introduction
Bob Symons died in Adelaide on 4 October 2006 after a long illness. He was distinguished through his contributions to our knowledge of the structure, function and replication of plant viruses, viroids and virusoids. His research culminated in the discovery of the hammerhead folding of the RNA chain and its role as a ribozyme in self-cleavage of the RNA in some of these plant pathogens. He was a leader in his field and was responsible for commercial applications of his research and the establishment in Adelaide of the first Australian company to produce and market molecular biologicals for research.
Early Years and Education
Robert (Bob) Henry Symons was born on 24 March 1934 on the family citrus block at Merbein in north-western Victoria. Bob’s paternal great-grandfather from Somerset had emigrated from England to Australia at the time of the gold rushes and established a family butchery business in Ballarat. Bob’s father, Henry Office Symons, was not interested in continuing in the business and at the conclusion of his education at Ballarat Grammar School moved to Merbein in 1921 and settled on a citrus and vines block named ‘Dalmura’. In 1927, he married Irene Olivette Wellington who also came from Ballarat. They had three children and Robert (in infancy known as ‘Bobbie’, later contracted to Bob) was the middle sibling, having younger and older sisters, Helen and Judith. Bob’s early years growing up on the citrus block were happy despite the Depression and the Second World War. He helped his father on the property, and he also owned a cow that he insisted on milking every morning before school. These childhood experiences ingrained in him a strong attachment to the land and were a strong influence on his subsequent involvement in agriculture and his life in agriculture-related scientific research.
After attending Merbein Primary School, Bob won a Government Scholarship in 1947 to Ballarat Boys’ Grammar School and remained there as a boarder until 1951 when he matriculated. The Principal at that time was Jack Dart whom Bob respected and admired. Bob would recall those years to friends and colleagues with memories good and not so good, recollections that included surviving the cold winters of Ballarat. The boredom away from home at weekends was offset by roaming nearby fields and gullies with the Principal’s dogs and rabbiting with a school friend, Ron Newland, who also came from Merbein. Bob recalled the boarding house food, the quality of which was limited by post-war rationing, and he blamed his subsequent poor dental health on all the bread and honey he consumed for much-needed calories. He was an excellent student and also participated in many sports, including being an expert rifleman. He became Dux of the school.
Bob had an interest in studying nuclear physics, but was persuaded by his father to take an Agricultural Science course, in which he enrolled at the University of Melbourne in 1952 as a resident in Trinity College. As expected, once he completed his degree in 1955, Bob joined his father in the family citrus business. Although he was much at home on the Merbein property and in driving a tractor, he gave much thought to his future over the ensuing year.
His attraction to creativity in science led to his travelling to Melbourne to survey the opportunities for undertaking a PhD degree, and in 1957 he began research under Professor Frank Hird in the Biochemistry Department at the University of Melbourne. He married Verna Lloyd in 1958, his wife of 48 years. Verna was a long-term friend and ‘the girl next door’—her family had citrus blocks on both sides of Bob’s father’s property at Merbein and the families were good friends. Thus, he began a career away from the family’s business, much as his father had moved from that of his family. From this stage in his life, Bob became committed to fundamental and applied research on plant pathogens.
Postdoctoral and Early Academic Career
After completing his PhD degree, Bob was awarded a CSIRO Postdoctoral Fellowship in 1961 to study at the Virus Research Unit in Cambridge, UK, with Roy Markham, an internationally recognised plant virologist at that time and head of the Unit. Bob’s interest in plant viruses was stimulated at that time and led to his establishing his career in that field. From England he was appointed to a lectureship in the Department of Agricultural Chemistry at the University of Adelaide’s Waite Agricultural Research Institute that he took up at the end of 1962. The Department was headed by Professor R. K. Morton, FAA, whom Bob had known during his time at the University of Melbourne where Morton had been Reader in Biochemistry. Just as Bob arrived Morton was offered the Chair of Biochemistry on the University’s main campus on North Terrace in central Adelaide and he moved across in early 1963. Bob moved with Morton and participated in the large task of building refurbishment, and in developing research programmes, funding and teaching. Progress was blighted by the death of Morton in September of that year as a result of a laboratory fire from an experiment he was conducting. Bob was working nearby in his own laboratory and rushed to the aid of Morton and his assistant. Bob was severely burned on the hands and arms while trying to quell the flames surrounding Morton, a humanitarian act that was never widely recognised. This tragic event was a dramatic blow to the extensive academic and infrastructural changes that Morton had set in train and also to Bob’s own academic plans. The destabilizing effect on the department took some time to overcome and was finally achieved after the appointment of W. H. Elliott to the Chair of Biochemistry in 1965.
The Adelaide Years
Family life
Bob spent the rest of his life in Adelaide. He settled with his family in Urrbrae, having built his home only a short walk from the Waite Institute, and they continued to live there despite Bob’s move to the North Terrace campus. Bob and Verna raised four children—two boys, Richard and Michael, and two girls, Helen and Alison—and together they enjoyed a full and vigorous life. Verna, a science graduate of the University of Melbourne, was a secondary school teacher until retiring age. Two children, Helen and Richard, became medical doctors; the younger son, Michael, became a winemaker; and the younger daughter, Alison, became an IT marketing manager. Bob had an early interest in wines, the growing of grapes and the virus diseases that afflict them. He maintained a large wine store beneath the lounge room of his home that provided much enjoyment and indeed amusement when guests realised they were sitting over many stacks of fine wines. In later years, Bob and Verna purchased a block of land in the Adelaide Hills and derived much pleasure from developing a vineyard that is still producing crops of Viognier and Shiraz grapes. Their closeness to the Waite Institute also led to their taking a particular interest in the arboretum there—Bob’s early agricultural background left him with an impressive ability to identify many eucalyptus species—and in the preservation of Urrbrae House that is situated near the Institute buildings and was once the home of the Institute’s Director; it is now a tourist attraction. Bob and Verna were both founding members of the Friends of Urrbrae House set up by the late Harold Woolhouse during his term as Director of the Waite.
The Adelaide Department of Biochemistry
At the University, Bob became a major member of an academic staff that grew following the appointment of Bill Elliott to eight lecturers plus postdoctoral fellows, with new support staff and rapidly developing Honours and PhD programmes. Bob’s contributions to the research and teaching strengths of the Department were recognised by his elevation to Senior Lecturer in 1967 and Reader in 1973, and to a Personal Chair in 1987. Bob supervised some 30 PhD students, many Honours students and a stream of postdoctoral fellows who formed part of his team over the years. His becoming a leader in his field resulted in many overseas visitors who spent their study leave with him. He remained close to the laboratory bench until in his later years his ill health made this no longer possible. His frequent presence in the laboratory ensured that his research students received excellent training and earned him their respect and admiration. It was well known that because of his personal scientific standards, he reacted severely to inadequate or sloppy technique or uncritical thinking. His teaching, like his research, focused on the molecular biology of viruses generally, but especially plant viruses, and on the much smaller infective molecules, viroids and virusoids. He was so informed in his subject that he lectured mostly without notes, a habit that impressed undergraduates and one that early in his career he had determined to follow after observing his PhD supervisor, Frank Hird, who practised the same lecturing mode.
During his years in Adelaide, Bob took up several research fellowship awards that enabled him to undertake study leave overseas and to take his family with him. With an NIH Fellowship he spent 1971 with Paul Berg at Stanford, a rewarding year in which, conjointly with Berg and Jackson, he accomplished the joining together of DNA molecules by enzymic ligation. (18) This was a milestone in the manipulation of DNA molecules and an essential step for the rapid development of molecular cloning by Cohen by 1972. In 1978, through the auspices of a Royal Society Bursary, Bob worked with Fred Sanger, Nobel Laureate at the Laboratory of Molecular Biology, Cambridge, UK, and was involved in the early stage of Sanger’s sequencing of mitochondrial DNA.
Through the course of his scientific life, Bob obtained research funding mainly from the Australian Research Council. In 1982, with three departmental colleagues (Elliott, Rogers and Wells), he obtained funding for one of the new Commonwealth Research Centres (CRCs), and a Special Research Centre for Gene Technology was formed in the Biochemistry Department. His share in this new type of high-level funding by the Australian Government gave a considerable boost to his plant virus research programmes over the next 8–9 years. It was followed by other major grants: a National Biotechnology Program Grant, 1984–1988 (with the CSIRO Division of Plant Industry in Canberra and the Institute of Medical and Veterinary Science in Adelaide), an ARC Special Centre for Basic and Applied Plant Molecular Biology, 1991– 1999 (with P. Langridge), and the Cooperative Research Centre for Viticulture, 1992–1999.
Bob’s international distinction in plant virology was recognised by several honours and awards. He was elected a Fellow of the Australian Academy of Science in 1983, Lemberg Medallist of the Australian Biochemical Society in 1985 and a Fellow of the Royal Society of London in 1988, and Macquarie University awarded him an honorary DSc degree in 1995. As noted earlier, he was promoted to a Personal Chair in 1987, and he was appointed Emeritus Professor of the University ofAdelaide in 2000.
Early Research
Bob’s research began in animal biochemistry at the University of Melbourne’s Biochemistry Department with Frank Hird, his supervisor. In ruminants, micro-organisms in the rumen produce short-chain fatty acids, especially butyrate, from glucose, and Bob studied the metabolism of butyrate in the gastrointestinal tract of sheep including the mechanism of formation of ketone bodies such as acetoacetic acid. (1–3) During those years, he maintained his interest in plants and plant diseases and that was firmly established when he spent two years in Cambridge studying plant viral ribonucleic acids with Roy Markham, one of the leaders in the field at the time. (5–7) In that period, 1961–1962, molecular biology was progressing rapidly and the puzzle of the triplet genetic code was becoming understood. On his arrival in Adelaide, Bob began his academic career furthering his commitment to plant viruses.
Although the main part of his career turned out to be directed to understanding the structure and function of viral nucleic acids in relation to the infectivity and development of plant diseases, he also carried two other research programmes from 1963 to 1978. One was to characterize the DNA component present in the lactate dehydrogenase–cytochrome b2 complex that had been isolated several years before from yeast and crystallized by R. K. Morton and colleagues. It was thought that the DNA may have some special cellular function, but in careful experiments that Bob conducted mostly with his PhD student L. A. Burgoyne, (8–14) it was conclusively demonstrated that the DNA was not a specific molecule, but a small degraded product from the yeast DNA and of variable composition.
In a totally different area, Bob published extensively on the mechanisms of the formation of peptide bonds on bacterial and mammalian ribosomes, mainly with PhD students Ray Harris and Julian Mercer and Postdoctoral Fellow Philip Greenwell in the years 1969–1978. (15–17,19,20) This part of his research portfolio necessitated significant skill in designing and conducting chemical syntheses and he had displayed those skills already in the synthesis of radioactive nucleotides described later. The work was directed to gaining knowledge of the mode of action of peptidyl transferase, the enzyme that is part of the 50S protein and RNA complexes of bacterial (and 60S mammalian) ribosomes and the binding sites for the participating tRNAs. Bob used the antibiotic puromycin that was known to terminate protein synthesis by blocking the activity of peptidyl transferase. He and his group prepared several analogues of puromycin and used these to measure their level of inhibition of peptidyltransferase activity and thereby obtain information about the active centre of the enzyme. It was known that the catalysis of peptide-bond formation occurred on the ribosome and involved the transfer of the nascent peptidyl-tRNA (donor substrate) in the P-site to aminoacyltRNA (acceptor substrate) in the A-site. From their findings, (21,22) Harris and Symons proposed a detailed model of the active centre of Escherichia coli peptidyltransferase in which there was a binding site for the 3/-terminal CpCpA of aminoacyl-and peptidyl-tRNA, present at each of the acceptor (A/) and donor (P/) sites respectively of the enzyme.
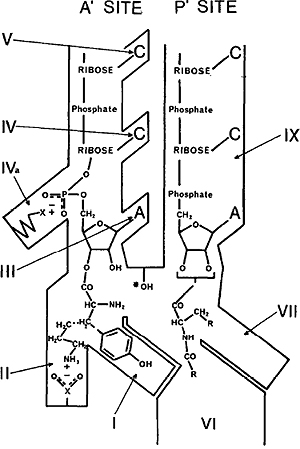
Figure 1. Diagrammatic representation of the active centre of peptidyl transferase on the E. coli ribosome. (21) The acceptor A/ site is where the aminoacyl–tRNA binds with its 3/ CCA terminus. The donor P/ site denotes the location of the extending peptidyl tRNA chain also with a 3/ CCA terminus. Functional groups and regions involved in the binding of the acceptor and receptor participants were deduced from studies of inhibitors and substrates and are denoted by I–IX. For example region I is hydrophobic and attracts aromatic amino-acyl groups to the A/ site whereas region II is hydrophilic and attracts tRNA with basic amino acids. Similar regions were mapped for the P/ site.
In particular, the acceptor CpCpA binding site was composed of sites for the terminal adenine, the first phosphoryl residue from the 3/-terminus, the 3/-penultimate cytosine, and the second 3/-CMP residue. In addition, two binding sites were present on each of the A/ and P/ sites, one for the basic and one for the hydrophobic aminoacyl-R groups of both aminoacyl-tRNA and the carboxyterminal amino acid of peptidyltRNA. Their improved model is shown in Figure 1.
Bob was aided in this work by Philip Greenwell, a bio-organic chemist from Oxford who was interested in locating the active sites of enzymes by affinity labelling. Chemically reactive analogues of substrates or other specific ligands, were synthesised and used to block the site specifically and irreversibly. (24–27) Greenwell’s recruitment to Bob’s group as a Queen Elizabeth II Fellow came about because he was at the Stanford Biochemistry Department with George Stark at the same time as Bob was there with Paul Berg. Philip relates that they first met because Bob was foraging around the department for suitable glassware with which to conduct his nucleotide syntheses, and Stark’s laboratory was the only one then doing any synthetic organic chemistry. In Adelaide, success was achieved with a puromycin derivative in which the chemically reactive group was positioned to attach in or near the binding site for the 3/-penultimate cytosine of the aminoacyltRNA. Bob’s group demonstrated not only that this affinity label specifically blocked the ability of the 50S subunits to synthesise peptide bonds, but also that once attached the molecule was authentically in situ, its amino acid moiety being able to act as acceptor in a single chain-terminating round of peptide synthesis. Furthermore, this specific labelling was found to be exclusively on the 23S rRNA rather than any of the 34 ribosomal proteins in the 50S subunit.
These findings, published in 1973 and 1974, allowed Bob’s group to conclude that the 23S rRNA has a direct role in peptidyl transferase activity and to speculate that this role might be binding of the 3/ CCA terminus of the tRNA by base-pairing as in double helices. That the affinity label had reacted with a single 23S rRNA G residue in the presumed binding site for the tRNA 3/-penultimate C residue was confirmed in 1978. (28,29) At this juncture, Bob concluded that further progress in ribosome studies would require structure-elucidating techniques that were being developed by large research groups in Europe and the USA and would be far beyond the resources of his laboratory. He had, however, made a significant contribution to understanding the nature and origin of the biochemical mechanism of protein synthesis. The ultimate results of his study of peptidyl transferase were amongst the earliest evidence in support of Francis Crick’s speculation that ‘it is tempting to wonder if the primitive ribosome could have been made entirely of RNA’ (Crick, 1968). Thirty years of widespread and intensive effort have shown that this was probably true. The formation of peptide bonds between amino acids is, indeed catalysed in the modern ribosome solely by 23S rRNA; in current parlance ‘the ribosome is a ribozyme’. Interestingly, Bob’s subsequent studies on plant virus RNA did much to demonstrate that certain RNA molecules can indeed exert catalytic activity.
The Preparation of 32P-Labelled Nucleotides
Bob’s experimental work on plant viruses, discussed later, from the very beginning included studies of RNA and DNA polymerases in viral replication. Such studies and the later work on viroids and virusoids demanded a constant and reliable supply of high-specific activity (>1 mC/µmole) 32P-labelled ribonucleoside di-and triphosphates and also the equivalent 32P-labelled deoxynucleoside di-and triphosphates and cyclic monophosphates. Purchases from overseas suppliers in the 1960s and 1970s were not only expensive, but the transport time to Australia meant loss of radioactivity half-life. Bob developed and improved the synthesis of a range of these compounds in his laboratory over a period of ten years and published a number of significant papers. (30–39) His success here is a fine example of his broad range of skills, from organic chemistry to viral biology. He not only supplied his personal research and that of his group with the 32P-labelled nucleotides by synthesising the nucleotides every second week, but he also provided these for the whole Biochemistry Department in which, at that time, research in molecular biology was rapidly increasing. As noted later, nucleotide synthesisand related methods formed the basis for the establishment of the Department-based and University-owned company Bresatec that supplied these materials nationally.
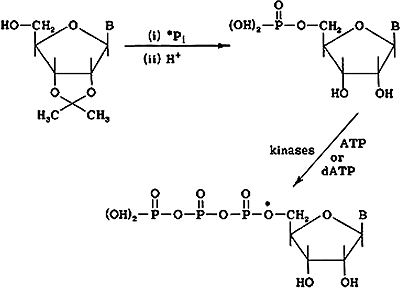
Figure 2. The synthetic pathways for 32P-labelled (A) deoxyribonucleotide and (B) ribonucleoside triphosphates. (37,38) B on the structures=base.
Bob devised a totally chemical method that he used only when his two-step procedure was unsuitable for a particular nucleotide. The two-step method involved the chemical synthesis of nucleoside 5/-[32P] monophosphates that were then converted to the triphosphates enzymically using the kinases, myokinase and pyruvate kinase. This method usually was more practicable, the yields of products were higher, of the order of 70–90% based on the input 32P, and had the advantage of being conducted in the same reaction flask, obviating the need to transfer highly radioactive material between vessels. For the synthesis of a labelled deoxyribonucleotide, the required deoxyribonucleoside was first treated with 32P-orthophosphoric acid under condensing conditions to produce phosphorylation of the 5/hydroxyl group. The 5/–32P-labelled deoxyribonucleotide product was then purified by chromatography followed by the final enzymic step to the triphosphate by addition of the diphosphate moiety using dATP as donor substrate and catalysed by a kinase. The synthesis of 32P-labelled ribonucleoside 5/triphosphates was similar except that the 2/,3/hydroxyl groups were protected from phosphorylation and the blocking group (O-isopropylidene) removed subsequent to the 5/ phosphorylation. The synthetic routes are shown in Figures 2A and 2B (37,38).
Plant Viruses and Viroids: Structure, Function and Replication
Bob chose to use Cucumber mosaic virus (CMV) as the main focus of his plant viral work. It is the type member of the genus Cucumovirus group and infects a number of important plants besides cucumber. Tomatoes infected with it develop yellowing, mottling and curling of the leaves. Bob used glasshouse facilities at the Waite Institute, 6 km from the Biochemistry Department but close to his home, to produce infected plants for the preparation of virus. He regularly stopped over at the Waite on his way to the main campus, while taking his children to school, to check on his plants.
Bob’s studies of CMV from 1963 to 1998 ranged from sequencing of the four viral RNAs that constitute the genome, and characterization of the satellite RNAs of the virus, to characterization of viral-induced polymerases in relation to viral replication. (61–81,94,95) Over that period, he published more than fifty papers with many students and postdoctoral fellows in international journals. A recent posthumous publication in this area was dedicated to Bob. (185)
During the course of his CMV work in the mid-1970s, Bob became interested in plant diseases such as Chrysanthemum stunt disease and Avocado sunblotch disease that were not caused by viruses. (82,84,89–91) Their etiology rests in infectious RNAs, the viroids, that only infect higher plants and that are much simpler than the viruses, consisting of a single circular RNA molecule that can vary in size from 246 to 400 nucleotides in length. They are rod-shaped molecules in which the RNA strands are double-stranded through internal base-pairing but with single-strand ‘loop outs’. Unlike plant viruses, viroids are not encapsidated in a protein coat and the RNAs are not translated into protein. The other small, rod-like RNA molecules that replicate in plants and that attracted Bob’s attention are the virusoids. They are also circular molecules, 324 to 388 nucleotides long, but are encapsidated in the virion of their helper virus and are known as satellite RNAs. They require their helper virus for replication and for provision of the protein coat. There are other satellite RNAs; for example, there is one associated with tobacco ringspot virus (sTRSV) that was characterized by some of Bob’s colleagues as an encapsidated circular RNA. When his research began, it was not known whether RNA-dependent RNA polymerases (RdRPs) were responsible for replication of viroids and virusoids. (93,94)
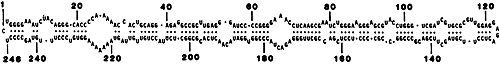
Figure 3. The 246 nucleotide sequence and postulated stable secondary structure of the fast variant of the four Cadang-Cadang viroids that causes the Cadang-Cadang disease of coconuts. (99) The rolling circle mechanism was proposed for the replication of this viroid.
Bob was intrigued by the infectivity of RNA molecules with such simple structures and thus began an adventure into understanding their nucleotide sequences, secondary structure and replication that ultimately led to the discovery of autocatalytic RNA or self-cleavage that occurs in some of these pathogens. Bob’s publications, some 45 papers, on viroid RNA sequences and secondary structures included studies not only of the Chrysanthemum and Avocado viroids, but also of those that cause Cadang-Cadang disease in coconut palms, (98,99) Citrus exocortis disease (96) and Lucerne transient streak disease. His paper with J. Haseloff and N. Mohamed99 on Cadang-Cadang disease showed that there were four RNAs, all of which are infectious. They were sequenced and their secondary structure and infectivity shown to be consistent with them being viroids, the four being identified as homologous variants with some variation between different isolates, two being smaller (for example, 246 [Fig. 3] and 287 nucleotides) and the others larger (for example, 492 and 574 nucleotides). These findings were judged of such importance that the RNA sequences were the front cover of the issue of Nature in which they were first published.
A major clue in the unfolding story of how viroids and virusoids replicate to produce more infectious particles was the finding that minus and plus RNA strands are present in infected plants. A rolling circle mechanism for replication of the circular RNAs catalysed by an in vivo RNA-dependent RNA polymerase (RdRP) had been proposed in the literature and by the 1970s, Bob had already begun studying virus-induced RdRPs. Two variations of the rolling circle mechanism can account for the formation of plus and minus strands. In one, the plus RNA is replicated by procession of the RdRP enzyme around the plus viroid template to form concatameric minus strands that are specifically cleaved and the minus viroid particles circularized by a host RNA ligase. (112–115) The RdRP then proceeds to produce a plus concatameric strand from each minus circle and the concatamers specifically cleave into plus unit length fragments that are then ligated to yield the pathogenic circular progeny. The other variation was that in the first copying step of the plus circle, the minus concatamer product is not cleaved but copied to a plus concatamer that is then specifically cleaved and the RNAs ligated to the circular progeny. Although the cleavage could be attributed to a plant ribonuclease, autocatalytic cleavage was shown to be responsible when Bob demonstrated that the RNAs from the replication of the viroid ASBV (Avocado sun-blotch) and several virusoids self-cleaved in the absence of any protein.
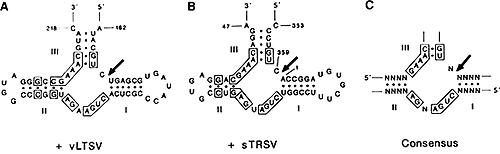
Figure 4. Comparisons of the two-dimensional hammerhead structures of the plus forms of: (A) encapsidated satellite virusoid RNA of lucerne transisent streak virus; (B) encapsidated linear satellite RNA tobacco ringspot virus; and (C) a consensus hammerhead sequence. (146) The self-cleaving sites are indicated by arrows.
The virusoids studied by the Symons group included several satellite RNAs of viruses, such as tobacco ring spot (sTRSV), Lucerne transient streak (vLTSV), Velvet tobacco mottle (vVTMoV), Solanum nodiflorum mottle (vSNMV) and subterranean clover mottle (vSCMoV). Their RNAs were sequenced and in 1986 Bob and colleagues showed (118–123) that the secondary structures of these transcripts included a folding that in two-dimensions resembled a hammerhead and contained the cleavage site (Fig. 4). The stability of the hammerhead structure comes from base pairing in three stems I, II and III, and a single-stranded region where the nucleoside cleavage site is on the 3/ side, usually of a cytosine residue. The self-cleavage mechanism is a magnesium-dependent transesterification that gives rise to fragments containing a 5/hydroxyl and a 2/,3/-cyclic phosphate. Comparisons of sequences revealed a consensus hammerhead (Fig. 4C).
Bob’s findings (129–143) were an extension of those of Thomas Cech at the University of Colorado, who by 1982 had discovered the self-cleavage of RNA that results in the removal of an intervening sequence from ribosomal RNA in the macronuclei of the protozoan Tetrahymena. A similar self-cleavage occurs with RNA transcripts from a satellite DNA found in the newt (salamander). Sidney Altman, Yale University, around the same time discovered that an RNA he called RNA-P, present in Escherichia coli, was responsible for the processing of the precursors of tRNAs, the RNAs involved in the synthesis of proteins. RNA-P brought about self-cleavage of the tRNA precursor.
These revolutionary findings established that although enzymes are usually proteins, some RNAs have catalytic activity. (146–149,154,168) Cech and Altman were awarded the Nobel Prize for chemistry in 1989 and catalytic RNAs were named ribozymes (see http://nobelprize.org/nobel_ prizes/chemistry/laureates/1989).
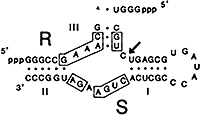
Figure 5. An example of a hammer-head that cleaves in trans. (129) The ribozyme sequence R binds to a 41 nucleotide substrate S. Arrow indicates self-cleaving site.
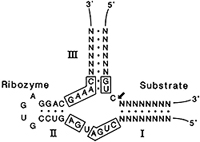
Figure 6. The consensus hammer-head structure designed by Haseloff and Gerlach for self-cleavage for ribozyme activity in trans (Haseloff and Gerlach, 1988).
In further studies, Bob and his coworkers demonstrated, in accord with findings of Uhlenbeck (Uhlenbeck, 1987), that ribozyme activity could occur in trans when two separate and independent molecules combined to form a hammerhead. One example was the cleavage of a substrate of 41 nucleotides by a separate fragment only 13 nucleotides long in which the RNA to be cleaved is separate from the ribozyme sequence. (129) This activity can occur, provided that the sequence of 13 conserved nucleotides (R) and the RNA substrate (S) of known sequence, such as a transcript of a gene, can form a hammerhead structure stabilized by the three base-paired stems (Fig. 5).
Bob suggested that such interactions may be important in gene regulation in normal cells as well as in the genesis of symptom expression on infection by RNA pathogens through the destruction of vital cellular RNAs. The whole story of the discovery of ribozymes and the possibility that they are vestiges of the pre-protein world was a dramatic finding in molecular biology and one to which Bob made an outstanding contribution. His studies of the hammerhead structure and particularly its potential for activity in trans led to the development of a potential method for cleaving RNAs in general and therefore the possibility of wide application. Jim Haseloff, a former PhD student of Bob’s, collaborated with W. L. Gerlach in CSIRO’s Division of Plant Industry and examined the self-cleavage properties of mutants of the hammerhead sequences. From the results, they proposed a generic structure for in trans ribozyme activity that would cleave a separate substrate (Fig. 6) and demonstrated that cleavage of a specific gene transcript could be obtained both in vitro and in vivo. The structure was patented by CSIRO and the name ‘Gene Shears’ was coined to indicate the potential for controlling the expression of genes in vivo (Haseloff and Gerlach, 1988). This ribozyme structure had an advantage over the American ones, in that it was more adaptable and had the potential to destroy any known RNA sequence—especially mRNAs involved in the causation of diseases in plants, animals and especially humans. The hammerhead ribozyme remains a candidate for the control of gene expression in health and disease but does not appear to have the same potential as other agents such as the in vivo RNA interference mechanism of gene control that occurs in cells as described in the late 1990s (Fire et al., 1998; Hamilton and Baulcombe, 1999) and for which Andrew Fire of Stanford University and Craig Mello of the University of Massachusetts, Boston, were awarded the Nobel Prize for Medicine in 2006.
Commercialization of Research and Advisory Roles
Bob’s development in 1966, for his own research, of methods to produce 32Plabelled ribonucleoside monophosphates efficiently with high specific activity led to his also supplying other research groups in the Biochemistry Department. Interest from around Australia to obtain these materials instead of from overseas became intense and led to the establishment of a University-owned company, BRESA, in 1982 to supply the products. The business expanded to embrace many other products for molecular biology research and in 1995 it was separated into two companies, BresaGen Ltd, that pursued basic research investigating stem cell therapy, and BresaTec Pty Ltd, that supplied oligonucleotides and radioactive nucleotides. Bob fostered the growth of these companies. He was Chairman of the Board and a Director of BRESA and of BresaTec in the period 1982–1987 and of BresaGen to 1996. In that year, BresaTec’s core business of synthesis and marketing of oligonucleotides and a range of other equipment and consumables for molecular biology separated as a privately owned company, GeneWorks Pty Ltd, that continues to the present time.
In 2003–2004, BresaGen altered its R&D theme and expanded its GMP fermentation facilities to the production of pharmaceutical proteins and peptides in E. coli and process development. BresaGen ceased trading in 2005 when it became Hospira Adelaide, which continues the business of process development of quality recombinant protein and peptide products.
As described below, after Bob’s move to the Waite Institute in 1991, he initiated another University-owned company, Waite Diagnostics, in 1997 that continues to provide routine diagnosis of grapevine diseases for the viticulture industry. Other commercial connections enjoyed by Bob were as a member of the Science Advisory Council of Calgene Pacific Pty Ltd and of the Scientific Advisory Council of Gene Shears Pty Ltd.
External Scientific Contributions
Bob filled several editorial positions over some thirty years, including Associate Editor of Virology and member of the editorial boards of Nucleic Acids Research, Plant Molecular Biology and RNA and of the advisory boards of Advances in Virus Research, The Plant Journal and Australian Journal of Grape and Wine Research.
Over the years he made significant contributions to the allocation of research funds, the development of research policy and the functioning of several research organizations. He was a member of the Working Parties on Biotechnology and on Higher Education Research Funding of the Australian Science and Technology Council (ASTEC) in 1982 and 1986, respectively. Over the period 1986–1991, he was appointed by CSIRO to act variously on the Advisory Committee, Division of Molecular Biology; the Committee for Review of Scientific Programs, Division of Molecular Biology; and the Advisory Committee, Division of Horticulture.
In the Australian Academy of Science, he was a foundation member of the Committee on Recombinant DNA Molecules (ASCORD) in 1975. He was elected a Fellow of the Academy in 1983 and served as a member of Council, 1997–1999.
Bob was a member, 1982–1986, of the Australian Industrial Research and Development Incentives Advisory Committee (AIRDIAC), an Australian Government body that was responsible for allocation of ‘Section 39’ Public Interest Grants. He played an important role, including Chairman 1989–1993, on the Biological Sciences Discipline Panel of the Australian Research Council.
At the Australian National University in the late 1980s, Bob was a member of the Review Committee of the Research School of Biological Sciences, of the Electoral Committees for the Chairs of Molecular and Evolutionary Biology and Molecular Biology, and of the Funding Cycle Review Committee.
Other positions he held in the 1990s included Chairman of the Board of the Australian Genome Research Facility and member of council of the Australian Wine Research Institute, based at Urrbrae in Adelaide.
Later Years at the Waite Agricultural Research Institute: the Wine Industry and Grape Diseases
Bob remained in the Biochemistry Department until 1991 when, at the age of 57, he decided to move his laboratory to the Department of Plant Science at the Waite Institute. His plant virus research had reached a stage where being within a plant science environment was an advantage, and he enjoyed a further eight years of productive research. His deep interest in wines and the wine industry took him in two directions. One was to maintain the small vineyard that he ran with the help of his wife and his winemaker son, Michael. The other was to initiate routine grapevine disease diagnosis that led to the establishment of the University of Adelaide company, Waite Diagnostics, that provides a service to grape growers in the control of grapevine pathogens. (189–191) On its establishment, the Grape and Vine Research and Development Council provided funds for R&D, and the service receives diagnostic requests from overseas including South Africa, Germany, the USA and New Zealand. This service continues to the present time under the management of one of Bob’s colleagues, Dr Nuredin Habili.
The highly sensitive molecular tools for diagnosing viral and viroid plant diseases are based on designing oligonucleotides that will specifically hybridize to the RNA or DNA of a particular pathogen. Detection of hybridization requires a traceable tag, but radioactively labelled probes are not stable or safe for routine diagnostic use and this stimulated interest in non-radioactive tags. Bob first utilized the biotin-avidinalkaline phosphatase system in which the biotinylation of DNA and RNA probes was achieved by photolysis of a biotin derivative, N-(4-azido–2-nitrophenyl)-N/(N-d-biotinyl-3-aminopropyl)-N/-methyl-1, 3-propanediamine (photobiotin) on to nucleotide probes. After dot-blot hybidization with photobiotin that had one biotin per 100–400 nucleotides, amounts of nucleic acid as low as 0.5 picograms could be detected, a sensitivity equivalent to what could be achieved by 32P-labelled probes. (43–51) The photobiotin probes were used until replaced by digoxygenin-labelling. It is pertinent to note that, as a result of Bob’s pioneering efforts in diagnostic technology, the avocado sun-blotch viroid has been eliminated from the plant sources and appears to be extinct in Australia.
In 1994, Bob became ill and was diagnosed with a pituitary tumour that was benign and successfully removed by surgery. He recovered well and returned to full-time work with his group. However, he developed a cerebral problem in 1996 that worsened over subsequent years and necessitated his retirement from laboratory work and finally from the Waite Institute in 2002. He was cared for at home in Urrbrae by Verna until hospitalization became inevitable. He died on 4 October 2006.
About this memoir
This memoir was originally published in Historical Records of Australian Science, vol.19, no.2, 2008. It was written by:
- George E. Rogers. Biochemistry Discipline, School of Molecular and Biomedical Science, The University of Adelaide, SA 5005, Australia. Corresponding author. Email: george.rogers@adelaide.edu.au
- William H. Elliott. Biochemistry Discipline, School of Molecular and Biomedical Science, The University of Adelaide, SA 5005, Australia.
Numbers in brackets refer to the bibliography.
Acknowledgements
The authors are deeply grateful for the help afforded us by Verna Symons and family and for the advice of some of Bob’s past colleagues, Professor John Randles and Drs Jane Visvader, Philip Greenwell, Peter Palukaitis and Nuredin Habili.
References
- Crick, F. H. C. (1968). The origin of the genetic code. J. Mol. Biol. 38, 367–379.
- Fire, A., S. Xu, M. K. Montgomery, S. A. Kostas, S. E. Driver, and C. C. Mello. (1998). Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391, 806–811.
- Hamilton, A. J., and D. C. Baulcombe. (1999). A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 286, 950–952.
- Haseloff, J., and W. L. Gerlach. (1988). Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature 334, 585–591.
- Uhlenbeck, O. C. (1987). A small catalytic oligoribonucleotide. Nature 328, 596–600.
Bibliography
- Hird, F. J. R. and Symons, R. H. (1959). The metabolism of glucose and butyrate by the omasum of the sheep. Biochim. Biophys.Acta 35, 422–434.
- Hird, F. J. R. and Symons, R. H. (1961). The mode of formation of ketone bodies from butyrate by tissue from the rumen and omasum of the sheep. Biochim. Biophys. Acta 46, 457–467.
- Hird, F. J. R. and Symons, R. H. (1962). The mechanism of ketone body formation from butyrate in rat liver. Biochem. J. 84, 212–216.
- Hird, F. J. R., Symons, R. H. and Weidemann, M. J. (1966). The effect of hexokinase and tricarboxylic acid cycle intermediates on fatty acid oxidation and formation of ketone bodies in rat liver mitochondria. Biochem. J. 98, 389–393.
- Reichmann, M. E., Rees, M. W., Symons, R. H. and Markham, R. (1962). Experimental evidence for the degeneracy of the nucleotide triplet code. Nature 195, 999–1000.
- Symons, R. H., Rees, M. W., Short, M. N. and Markham, R. (1963). Relationships between the ribonucleic acid and protein of some plant viruses. J. Mol. Biol. 6, 1–15.
- Symons, R. H. (1963). Genetic coding in plant and bacterial viruses. Rev. Pure Appl. Chem. 13, 211–246.
- Symons, R. H. (1965). The DNA component of cytochrome b2. 1. Isolation of b2-DNA and the behaviour of cytochrome b2 during chromatography on DEAE-cellulose and sedimentation in a sucrose gradient. Biochim. Biophys. Acta 103, 298–310.
- Ellery, B. W. and Symons, R. H. (1966). Loss of adenine during the hydrazine degradation of DNA. Nature 210, 1159–1160.
- Nicholls, R. G., Atkinson, M. R., Burgoyne, L. A. and Symons, R. H. (1966). Changes in the properties of Lactate dehydrogenase (cytochrome b2) from yeast during preparation of the crystalline enzyme. Biochim. Biophys. Acta 122, 14–21.
- Symons, R. H. and Burgoyne, L. A. (1966). Lactate (cytochrome) dehydrogenase (crystalline, yeast). Methods in Enzymology 9, 314–321.
- Burgoyne, L. A. and Symons, R. H. (1966). The DNA component of cytochrome b2. II. The specificity of its association with the enzyme and its origin from high molecular weight DNA. Biochim. Biophys. Acta 129, 502–510.
- Symons, R. H. and Ellery, B. W. (1967). The DNA component of cytochrome b2. III. Base sequence studies on preparations of yeast DNA. Biochim. Biophys. Acta 145, 368–377.
- Burgoyne, L. A., Dyer, P. Y. and Symons, R. H. (1967). On the molecular structure of crystalline yeast cytochrome b2. J. Ultrastructure Res. 20, 20–32.
- Symons, R. H., Harris, R. J., Clarke, L. P., Wheldrake, J. F. and Elliott, W. H. (1969). Structural requirements for inhibition of polyphenylalanine synthesis by aminoacyl and nucleotidyl analogues of puromycin. Biochim. Biophys. Acta 179, 248–250.
- Harris, R. J., Hanlon, J. E. and Symons, R. H. (1971). Peptide bond formation on the ribosome. Structural requirements for inhibition of protein synthesis and of release of peptides from peptidyl-tRNA on bacterial and mammalian ribosomes by aminoacyl and nucleotidyl analogues of puromycin. Biochim. Biophys. Acta 240, 244–262.
- Mercer, J. F. B. and Symons, R. H. (1971). The use of EEDQ (N-ethoxycarbonyl-2ethoxy-l,2dihydroquinoline) in the selective N4-acylation of cytidine and its derivatives. Biochim. Biophys. Acta 238, 27–30.
- Jackson, D. A., Symons, R. H. and Berg, P. (1972). Biochemical method for inserting new genetic information into DNA of simian virus 40: Circular SV40 DNA molecules containing lambda phage genes and the galactose operon of Escherichia coli. Proc. Natl. Acad. Sci. USA 69, 2904–2909.
- Harris, R. J., Mercer, J. F. B., Skingle, D. C. and Symons, R. H. (1972). Substrates for ribosomal peptidyl transferase: Synthesis of 3/-N-aminoacyl and 5/-O-nucleotidyl analogues of puromycin. Canad. J. Biochem. 50, 918–926.
- Mercer, J. F. B. and Symons, R. H. (1972). Peptidyl donor substrates for ribosomal peptidyl transferase: Chemical synthesis and biological activity of N-acetyl aminoacyl di-and trinucleotides. European J. Biochem. 28, 38–45.
- Harris, R. J. and Symons, R. H. (1973). On the molecular mechanism of action of certain substrates and inhibitors of ribosomal peptidyl transferase. Bioorganic Chem. 2, 266–285.
- Harris, R. J. and Symons, R. H. (1973). A detailed model of the active centre of Escherichia coli peptidyl transferase. Bioorganic Chem. 2, 286–292.
- Harris, R. J., Greenwell, P. and Symons, R. H. (1973). Affinity labelling of ribosomal peptidyl transferase by a puromycin analogue. Biochem. Biophys. Res. Commun. 55, 117–124.
- Eckermann, D. J., Greenwell, P. and Symons, R. H. (1974). Peptide bond formation on the ribosome. A comparison of the acceptor substrate specificity of peptidyl transferase in bacterial and mammalian ribosomes using puromycin analogues. Eur. J. Biochem. 41, 547–554.
- Vanin, E. F., Greenwell, P. and Symons, R. H. (1974). Structure-activity relationships of puromycin analogues on Escherichia coli polysomes. FEBS Letters 40, 124–126.
- Greenwell, P., Harris, R. J. and Symons, R. H. (1974). Affinity labelling of 23S ribosomal RNA in the active centre of Escherichia coli peptidyl transferase. Eur. J. Biochem. 49, 539–554.
- Vanin, E. F. and Symons, R. H. (1976). The preparation of high specific activity [3H]chloramphenicol base and chloramphenicol labelled in the propanediol side-chain. Anal. Biochem. 76, 259–268.
- Symons, R. H., Harris, R. I., Greenwell, P., Eckermann, D. J. and Vanin, E. F. (1978). The use of puromycin analogs and related compounds to probe the active center of peptidyl transferase on Escherichia coli ribosomes. Bioorganic Chemistry, vol. IV, pp. 409–436 (Academic Press).
- Eckermann, D. J. and Symons, R. H. (1978). Sequence at the site of attachment of an affinity-label derivative of puromycin on 23S ribosomal RNA of Escherichia coli ribosomes. Eur. J. Biochem. 82, 225–234.
The preparation of 32P-labelled nucleotides
- Greenlees, A. W. and Symons, R. H. (1966). The preparation of 32P-labelled nucleoside 5/-monophosphates. Biochim. Biophys. Acta 119, 241–248.
- Symons, R. H. (1966). A rapid improved method for the synthesis of 32P-labelled ribonucleoside 5/-monophosphates. Biochem. Biophys. Res. Commun. 24, 972–976.
- Symons, R. H. (1968). Modified procedure for the synthesis of 32P-Iabelled ribonucleoside 5/-monophosphates of high specific activity. Biochim. Biophys. Acta 155, 609–610.
- Symons, R. H. (1969). Preparation of α–32P-nucleoside and deoxynucleoside 5/triphosphates from 32Pi and protected and unprotected nucleosides. Biochim. Biophys. Acta 190, 548–550.
- Symons, R. H. (1970). Practical methods for the routine chemical synthesis of 32P-labelled nucleoside di-and triphosphates. Biochim. Biophys. Acta 209, 296–305.
- Symons, R. H. (1970). 32P-3/,5/-Cyclic AMP: A simple preparative procedure. Biochem. Biophys. Res. Commun. 38, 807–810.
- Symons, R. H. (1973). Improved synthesis of 32P-3/,5/-cyclic AMP, cyclic GMP and other 3/,5/-cyclic ribo-and deoxyribonucleotides of high specific activity. Biochim. Biophys. Acta 320, 535–539.
- Symons, R. H. (1974). Synthesis of α– 32P-ribo-and deoxyribonucleoside 5/triphosphates. Methods in Enzymology 29, 102–115.
- Symons, R. H. (1974). The synthesis of 32Padenosine-3/,5/-cyclic phosphate and other ribo-and deoxyribonucleoside-3/,5/-cyclic phosphates. Methods in Enzymology 38, 410–420.
- Symons, R. H. (1977). The rapid, simple and improved preparation of high specific activity α[32P]ATP and α[32P] ATP. Nucleic Acids Res. 4, 4347–4355.
- Palukaitis, P., Rakowski, A. G., Alexander, D. McE. and Symons, R. H. (1981). Rapid indexing of the sunblotch disease of avocados using a complementary DNA probe to avocado sunblotch viroid. Ann.Appl. Biol. 98, 439–449.
- Symons, R. H. (1984). Diagnostic approaches for the rapid and specific detection of plant viruses and viroids. In Plant-Microbe Interactions: Molecular and Genetic Perspectives, ed. T. Kosuge and E.W. Nester, vol. 1, pp. 93–124 (Macmillan Publishing Co., New York).
- Barker, J. M., McInnes, J. L., Murphy, P. J. and Symons, R. H. (1985). Dot-blot procedure with 32P-DNA probes for the sensitive detection of avocado sunblotch and other viroids in plants. J. Virol. Methods 10, 87–98.
- Symons, R. H. (1985). New developments in the use of DNA probes for the rapid detection of viral pathogens. In Pests and Parasites as Migrants: An Australian Perspective, ed. A. Gibbs and R. Meischke, pp. 85–90 (Australian Academy of Science).
- Forster, A. C., McInnes, J. L., Skingle, D. C. and Symons, R. H. (1985). Non-radioactive hybridization probes prepared by the chemical labelling of DNA and RNA with a novel reagent, Photobiotin. Nucleic Acids Res. 13, 745–761.
- Rezaian, M. A. and Symons, R. H. (1986). Anti-sense regions in satellite RNA of cucumber mosaic virus form stable complexes with the viral coat protein gene. Nucleic Acids Res. 14, 3229–3239.
- Habili, N., McInnes, J. L. and Symons, R. H. (1987). Non-radioactive, Photobiotin-labelled DNA probes for the routine diagnosis of barley yellow dwarf virus. J. Virol. Methods 16, 225–237.
- Li, P., Medon, P. P., Skingle, D. C., Lancer, J. A. and Symons, R. H. (1987). Enzyme-linked synthetic oligonucleotide probes in the detection of enterotoxigenic Escherichia coli in faecal specimens. J. Clin. Microbiol. 15, 5275–5287.
- McInnes, J. L., Vize, P. D., Habili, N. and Symons, R. H. (1987). Chemical biotinylation of nucleic acids with the novel reagent photobiotin and their use as hybridization probes. Focus 9(4), 1–4.
- Medon, P. P., Lanser, J. A., Monckton, P. P., Li, P. and Symons, R. H. (1988). Identification of enterotoxigenic Escherichia coli from clinical specimens with enzyme-labelled synthetic oligonucleotide probes. J. Clin. Microbiol. 26, 2173–2176.
- McInnes, J. L., Forster, A. C. and Symons, R. H. (1988). Photobiotin-labelled DNA and RNA hybridization probes. Methods in Molecular Biology 3, 401–414.
- McInnes, J. L. and Symons, R. H. (1989). Enzymatic and chemical techniques for labelling nucleic acids with radioisotopes. In Nucleic Acid Probes, ed. R. H. Symons, pp. 1–31 (CRC Press).
- McInnes, J. L. and Symons, R. H. (1989). Preparation and detection of non-radioactive nucleic acid and oligonucleotide probes. In Nucleic Acid Probes, ed. R. H. Symons, pp. 33–80 (CRC Press).
- McInnes, J. L. and Symons, R. H. (1989). Nucleic Acid Probes in the diagnosis of plant viruses and viroids. In Nucleic Acid Probes, ed. R. H. Symons, pp. 113–138 (CRC Press).
- McInnes, J. L., Habili, N. and Symons, R. H. (1989). Non-radioactive, photobiotinlabelled DNA probes for routine diagnosis of viroids in plant extracts. J.Virol. Methods 23, 299–312.
- McInnes, J. L., Forster, A. C., Skingle, D. C. and Symons, R. H. (1990). Preparation and use of photobiotin. Methods Enzymol. 184, 588–600.
- McInnes, J. L. and Symons, R. H. (1991). Photobiotin labelling of DNA and RNA hybridization probes. In Methods in Gene Technology, ed. J. W. Dale and P. G. Sanders, vol. 1, pp. 109–125.
Plant viruses and viroids: structure, function and replication
- Panter, R. A. and Symons, R. H. (1966). Isolation and properties of a DNA-containing rod-shaped bacteriophage. Aust. J. Biol. Sci. 19, 565–573.
- Gilliland, J. M., Langman, R. E. and Symons, R. H. (1966). Properties of the rionucleotide kinases following infection of cucumbers with tobacco ringspot virus. Virology 30, 716–723.
- Gilliland, J. M. and Symons, R. H. (1967). Partial purification and properties of ribonucleotide kinases in virus infected and healthy plants. Virology 33, 221–226.
- Harris, R. J., Panter, R. A. and Symons, R. H. (1968). Metabolism of deoxythymidine 3/mono-and diphosphate in normal and bacteriophage T4-infected Escherichia coli. Biochim. Biophys. Acta 161, 291–298.
- May, J. T. and Symons, R. H. (1968). Properties and intracellular distribution of nucleoside diphosphokinases from cucumber cotyledons. Phytochemistry 7, 1271–1278.
- Gilliland, J. M. and Symons, R. H. (1968). Properties of a plant virus-induced RNA polymerase in cucumbers infected with cucumber mosaic virus. Virology 36, 232–240.
- May, J. T., Gilliland, I. M. and Symons, R. H. (1969). Plant virus-induced RNA polymerase. Properties of the enzyme partly purified from cucumber cotyledons infected with cucumber mosaic virus. Virology 39, 54–65.
- May, J. T., Gilliland, J. M. and Symons, R. H. (1970). Properties of a plant virus-induced RNA polymerase in particulate fractions of cucumbers infected with cucumber mosaic virus. Virology 41, 653–664.
- May, J. T. and Symons, R. H. (1971). Specificity of the cucumber mosaic virus-induced RNA polymerase for RNA and polynucleotide templates. Virology 44, 517–526.
- Peden, K. W. C., May, J. T. and Symons, R. H. (1972). A comparison of two plant virus-induced RNA polymerases. Virology 47, 498–501.
- Peden, K. W. C. and Symons, R. H. (1973). Cucumber mosaic virus contains a functionally divided genome. Virology 53, 487–492.
- Clark, G. L., Peden, K. W. C. and Symons, R. H. (1974). Cucumber mosaic virus-induced RNA polymerase: partial purification and properties of the template-free enzyme. Virology 62, 434–443.
- Schwinghamer, M. W. and Symons, R. H. (1975). Fractionation of cucumber mosaic virus RNA and its translation in a wheat embryo system. Virology 63, 252–262.
- Symons, R. H. (1975). Cucumber mosaic virus RNA contains 7-methyl guanosine at the 5/-terminus of all four RNA species. Mol. Biol. Reports 2, 277–285.
- Schwinghamer, M. W. and Symons, R. H. (1977). Translation of the four major RNA species of cucumber mosaic virus in plant and animal cell-free systems and in toad oocytes. Virology 79, 88–108.
- Gould, A. R. and Symons, R. H. (1977). Determination of the sequence homology between the four RNA species of cucumber mosaic virus by hybridization analysis with complementay DNA. Nucleic Acids Res.4, 3787–3802.
- Symons, R. H. (1978). The two-step purification of ribosomal RNA and plant viral RNA by polyacrylamide slab gel electrophoresis. Aust. J. Biol. Sci. 3, 25–37.
- Gould, A. R., Palukitis, P., Symons, R. H. and Mossop, D. W. (1978). Characterization of a satellite RNA associated with cucumber mosaic virus. Virology 84, 443–455.
- Gonda, T. J. and Symons, R. H. (1978). The use of hybridization analysis with complementary DNA to determine the RNA sequence homology between strains of plant viruses: Its application to several strains of cucumoviruses. Virology 88, 361–370.
- Gould, A. R. and Symons, R. H. (1978). Alfalfa mosaic virus RNA. Determination of the sequence homology between the four RNA species and a comparison with the four RNA species of cucumber mosaic virus. Eur. J. Biochem. 91, 269–278.
- Palukaitis, P. and Symons, R. H. (1978). Synthesis and characterization of a complementary DNA probe for chrysanthemum stunt viroid. FEBS Letters 92, 268–272.
- Kumarasamy, R. and Symons, R. H. (1979). Extensive purification of the cucumber mosaic virus-induced RNA replicase. Virology 96, 622–632.
- Gonda, T. J. and Symons, R. H. (1979). Cucumber mosaic virus replication in cow-pea protoplasts: Time course of virus, coat protein and RNA synthesis. J. Gen.Virol. 45, 723–736.
- Symons, R. H. (1979). Extensive sequence homology at the 3/-termini of the four RNAs of cucumber mosaic virus. Nucleic Acids Res. 7, 825–837.
- Palukaitis, P. and Symons, R. H. (1979). Hybridization analysis of chrysanthemum stunt viroid with complementary DNA and the quantitation of viroid RNA sequences in extracts of infected plants. Virology 98, 238–245.
- Palukaitis, P., Hatta, T., Alexander, D. and Symons, R. H. (1979). Characterization of a viroid associated with avocado sunblotch disease. Virology 99, 145–151.
- Kumarasamy, R. and Symons, R. H. (1979). The tritium labelling of small amounts of protein for analysis by electrophoresis on sodium dodecylsulphate polyacrylamide slab gels. Anal. Biochem. 95, 359–363.
- Palukaitis, P. and Symons, R. H. (1980). Purification and characterization of the circular and linear forms of chrysanthemum stunt viroid. J. Gen. Virol. 46, 477–489.
- Gunn, M. R. and Symons, R. H. (1980). Sequence homology at the 3/-termini of the four RNAs of alfalfa mosaic virus. FEBS Letters 109, 145–150.
- Gunn, M. R. and Symons, R. H. (1980). The RNAs of Bromoviruses: 3/-Terminal sequences of the four brome mosaic virus RNAs and comparison with cowpea chlorotic mottle virus RNA 4. FEBS Letters 115, 77–82.
- Molloy, P. L. and Symons, R. H. (1980). Cleavage of DNA-RNA hybrids by Type II restriction enzymes. Nucleic Acids Res.8, 2939–2946.
- Palukaitis, P. and Symons, R. H. (1980). Nucleotide sequence homology of thirteen tobamovirus RNAs as determined by hybridization analysis with complementary DNA. Virology 107, 354–361.
- Haseloff, J. and Symons, R. H. (1981). Chrysanthemum stunt viroid: Primary sequence and secondary structure. Nucleic Acids Res. 9, 2741–2752.
- Allen, R. N., Palukaitis, P. and Symons, R. H. (1981). Purified avocado sunblotch viroid causes disease in avocado seedlings. Aust. Plant Path. 10, 31–32.
- Symons, R. H. (1981). Avocado sunblotch viroid: Primary sequence and proposed secondary structure. Nucleic Acids Res.9, 6527–6537.
- Wilson, P. A. and Symons, R. H. (1981). The RNAs of cucumoviruses: 3/-Terminal sequence analysis of two strains of tomato aspermy virus. Virology 112, 342–345.
- Gill, D. S., Kumarasamy, R. and Symons, R. H. (1981). Cucumber mosaic virus-induced RNA replicase: Solubilization and partial purification of the particulate enzyme. Virology 113, 1–8.
- Gordon, K. H. J., Gill, D. S. and Symons, R. H. (1982). Highly purified cucumber mosaic virus-induced RNAdependent RNA polymerase does not contain full length translation products of the genomic RNAs. Virology 123, 284–295.
- Gould, A. R. and Symons, R. H. (1982). Cucumber mosaic virus RNA 3. Determination of the nucleotide sequence provides the amino acid sequences of protein 3A and viral coat protein. Eur. J. Biochem. 126, 217–226.
- Visvader, J. E., Gould, A. R., Bruening, G. E. and Symons, R. H. (1982). Citrus exocortis viroid: sequence and secondary structure of an Australian isolate. FEBS Letters 137, 288–292.
- Haseloff, J. and Symons, R. H. (1982). Comparative sequence and structure of viroid-Iike RNAs of two plant viruses. Nucleic Acids Res. 10, 3681–3691.
- Mohamed, N. A., Haseloff, J., Imperial, J. S. and Symons, R. H. (1982). Characterization of the different electrophoretic forms of the cadang-cadang viroid. J. Gen. Virol. 63, 181–188.
- Haseloff, J., Mohamed, N. A. and Symons, R. H. (1982). Viroid RNAs of the cadang-cadang disease of coconuts. Nature 299, 316–321.
- Bruening, G. E., Gould, A. R., Murphy, P. J. and Symons, R. H. (1982). Oligomers of avocado sunblotch viroid are found in infected avocado leaves. FEBS Letters 148, 71–78.
- Dale, J. L., Allen, R. N. and Symons, R. H. (1982). Avocado sunblotch viroid. CMI/AAB Descriptions of Plant Viruses, No. 254.
- Gordon, K. H. J. and Symons, R. H. (1983). Satellite RNA of cucumber mosaic virus forms a secondary structure with partial 3/-terminal homology to genomal RNAs. Nucleic Acids Res. 11, 947–960.
- Symons, R. H., Gill, D. S., Gordon, K. H. J. and Gould, A. R. (1983). Gene content and expression of the four RNAs of cucumber mosaic virus. In Manipulation and Expression of Genes in Eukaryotes, ed. P. Nagley, A. W. Linnane, W. J. Peacock and J. A. Pateman, pp. 373–380 (Academic Press, Sydney).
- Symons, R. H. (1983). A molecular biological approach to relationships among viruses. Ann. Rev. Phytopathol. 21, 179–199.
- Visvader, J. E. and Symons, R. H. (1983). Comparative sequence and structure of different isolates of citrus exocortis viroid. Virology 130, 232–237.
- Keese, P., Bruening, G. and Symons, R. H. (1983). Comparative sequence and structure of circular RNAs from two isolates of lucerne transient streak virus. FEBS Letters 159, 185–190.
- Rezaian, M. A., Williams, R. H. V., Gordon, K. H. J., Gould, A. R. and Symons, R. H. (1984). Nucleotide sequence of cucumber mosaic virus RNA 2 reveals a translation product significantly homologous to corresponding proteins of other viruses. Eur. J. Biochem. 143, 277–284.
- Symons, R. H. (1985). Viral genome structure. In The Plant Viruses, ed. R. I. B. Francki, vol. 1, pp. 57–81 (Plenum Publishing Corp.).
- Gordon, K. H. J. and Symons, R. H. (1985). Subgenomic RNAs with nucleotide sequences derived from RNAs 1 and 2 of cucumber mosaic virus can act as messenger RNAs in vitro. Virology 142, 144–158.
- Rezaian, M. A., Williams, R. H. V. and Symons, R. H. (1985). Nucleotide sequence of cucumber mosaic virus RNA 1: Presence of a sequence complementary to part of the viral satellite RNA and homologies with other viral RNAs. Eur. J. Biochem. 150, 331–339.
- Jaspars, E. M. J., Gill, D. S. and Symons, R. H. (1985). Viral RNA synthesis by a particulate fraction from cucumber seedlings infected with cucumber mosaic virus. Virology 144, 410–425.
- Hutchins, C. J., Keese, P., Visvader, J. E., Rathjen, P. D., McInnes, J. L. and Symons, R. H. (1985). Comparison of multimeric plus and minus forms of viroids and virusoids. Plant Mol. Biol. 4, 293–304.
- Keese, P. and Symons, R. H. (1985). Domains in viroids: Evidence of intermolecular RNA rearrangements and their contribution to viroid evolution. Proc. Natl. Acad. Sci. USA 82, 4582–4586.
- Visvader, J. E. and Symons, R. H. (1985). Eleven new sequence variants of citrus exocortis viroid and the correlation of sequence with pathogenicity. Nucleic Acids Res. 13, 2907–2920.
- Visvader, J. E., Forster, A. C. and Symons, R. H. (1985). Infectivity and in vitro mutagenesis of monomeric cDNA clones of citrus exocortis viroid indicates the site of processing of viroid precursors. Nucleic Acids Res. 13, 5843–5856.
- Symons, R. H., Haseloff, J., Visvader, J. E., Keese, P., Murphy, P. J., Gill, D. S., Gordon, K. H. J. and Bruening, G. (1985). On the mechanism of replication of viroids, virusoids and satellite RNAs. In Subviral Pathogens of Plants and Animals: Viroids and Prions, ed. K. Maramorosch and J. J. McKelvey, pp. 235–263 (Academic Press).
- Visvader, J. E. and Symons, R. H. (1986). Replication of in vitro-constructed viroid mutants: Location of the pathogenicity modulating domain of citrus exocortis viroid. EMBO J. 5, 2051–2055.
- Hutchins, C. J., Rathjen, P. D., Forster, A. C. and Symons, R. H. (1986). Self-cleavage of plus and minus RNA transcripts of avocado sunblotch viroid. Nucleic Acids Res. 14, 3627–3640.
- Forster, A. C. and Symons, R. H. (1987). Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell 49, 211–220.
- Forster,A.C.and Symons,R.H.(1987).Self cleavage of virusoid RNA is performed by the proposed 55-nucleotide active site. Cell 50, 9–16.
- Forster, A. C., Jeffries, A. C., Sheldon, C. C. and Symons, R. H. (1987). Structural and ionic requirements for self-cleavage of virusoid RNAs and trans self-cleavage of viroid RNA. Cold Spring Harbour Symp. Quant. Biol. 52, 249–259.
- Keese, P. and Symons, R. H. (1987). The structure of viroids and virusoids. In Viroids and Viroid-like Pathogens, ed. J. S. Semancik, pp. 1–47 (Academic Press).
- Keese, P. and Symons, R. H. (1987). Physical-chemical properties: Molecular structure (primary and secondary). In The Viroids, ed. T. O. Diener, pp. 37–62 (Plenum Publishing Corporation).
- Symons, R. H., Hutchins, C. J., Forster, A. C., Rathjen, P. D., Keese, P. and Visvader, J. E. (1987). Self-cleavage of RNA in the replication of viroids and virusoids. J. Cell Sci. Suppl. 7, 303–318.
- Keese, P., Visvader, J. E. and Symons, R. H. (1988). Sequence variability and structure/ function relationships of viroids. In RNA Genetics, Volume 3: RNA Replication, ed. E. Domingo, J. Holland and P. Ahlquist, pp. 71–98 (CRC Press).
- Keese, P., Osorio-Keese, M. E. and Symons, R. H. (1988). Coconut tinanjaga viroid: Sequence homology with coconut cadang-cadang viroid and other potato spindle tuber viroid related RNAs. Virology 162, 508–510.
- Forster, A. C., Davies, C., Sheldon, C. C., Jeffries, A. C. and Symons, R. H. (1988). Self-cleaving viroid and newt RNAs may only be active as dimers. Nature 334, 265–267.
- Davies, C. and Symons, R. H. (1988). Further implications for the evolutionary relationships between tripartite plant viruses based on cucumber mosaic virus RNA 3. Virology 165, 216–224.
- Jeffries, A. C. and Symons, R. H. (1989). A catalytic 13-mer ribozyme. Nucleic Acids Res. 17, 1371–1377.
- Sheldon, C. C. and Symons, R. H. (1989). RNA stem stability in the formation of a self-cleaving hammerhead structure. Nucleic Acids Res. 17, 5665–5677.
- Sheldon, C. C. and Symons, R. H. (1989). Mutagenesis analysis of a self-cleaving RNA. Nucleic Acids Res. 17, 5679–5685.
- Rakowski, A. G. and Symons, R. H. (1989). Comparative sequence studies of variants of avocado sunblotch viroid. Virology 173, 352–356.
- Symons, R. H. (1989). Pathogenesis by antisense. Nature 338, 542–543.
- Symons, R. H. (1989). Self-cleavage of RNA in the replication of small pathogens of plants and animals. Trends Biochem. Sci. 14, 445–450.
- Habili, N. and Symons, R. H. (1989). Evolutionary relationship between luteoviruses and other RNA plant viruses based on sequence motifs in their putative RNA polymerases and nucleic acid helicases. Nucleic Acids Res. 17, 9543–9555.
- Symons, R. H. (1990). The fascination of low molecular weight pathogenic RNAs. Seminars in Virology, ed. R. H. Symons, vol. 1, pp. 75–81.
- Symons, R. H. (1990). Self-cleavage of RNA in the replication of viroids and virusoids. Seminars in Virology, ed. R. H. Symons, vol. 1, pp. 117–126.
- Forster, A. C., Davies C., Hutchins, C. and Symons R. H. (1990). Characterisation of self-cleavage of viroid and virusoid RNAs. Methods in Enzymology 181, 581–607.
- Sheldon, C. C., Jeffries, A. C., Davies, C. and Symons, R. H. (1990). RNA self-cleavage by the hammerhead structure. Nucleic Acids and Molecular Biology 4, 227–242.
- Davies, C., Haseloff, J. and Symons, R. H. (1990). Structure, self-cleavage and replication of two viroid-like satellite RNAs (virusoids) of subterranean clover mottle virus. Virology 177, 216–224.
- Skingle, D. C., McInnes, J. L. and Symons, R. H. (1990). An improved method for eliminating RNA contamination of plasmid DNA preparations. Biotechniques 9, 314–317.
- Symons, R. H. (1991). The intriguing viroids and virusoids: What is their information content and how did they evolve? Mol. Plant Microbe Interactions 4, 111–121.
- Davies, C., Sheldon, C. C. and Symons, R. H. (1991). Alternative hammerhead structures in the self-cleavage of avocado sunblotch viroid RNAs. Nucleic Acids Res. 19, 1893–1898.
- Symons, R. H. (1991). Ribozymes. Crit. Rev. Plant Sci. 10, 189–234.
- McInnes, J. L. and Symons, R. H. (1991). Comparative structure of viroids and their rapid detection using radioactive and nonradioactive Nucleic Acid Probes. In Viroids: Pathogens at the Frontier of Life, ed. K. Maramorosch, pp. 21–58 (CRC Press).
- Symons, R. H. (1992). Small catalytic RNAs. Ann. Rev. Biochem. 61, 641–671.
- Semancik, J. S., Szychowski, J. A., Rakowski, A. G. and Symons, R. H. (1993). Isolates of citrus exocortis viroid recovered by host and tissue selection. J. Gen. Virol. 74, 2427–2436.
- Sheldon, C. C. and Symons, R. H. (1993). Is hammerhead self-cleavage involved in the replication of a virusoid in vivo? Virology 194, 463–474.
- Symons, R. H. (1994). A plant molecular virologist’s view of an intriguing virus and its catalytic RNA. In The Unique Hepatitis Delta Virus, ed. G. Dinter-Gottlieb, pp. 1–10 (R. G. Landes Company).
- Bonfiglioli, R. G., McFadden, G. I. and Symons, R. H. (1994). In situ hybridization localises avocado sunblotch viroid on chloroplast thylakoid membranes and coconut cadang cadang viroid in the nucleus. The Plant Journal 6, 99–103.
- Hodgson, R. A. J., Shirley, N. S. and Symons, R. H. (1994). Probing the hammerhead ribozyme structure with ribonucleases. Nucleic Acids Res. 22, 1620–1625.
- Semancik,J.S.,Szychowski,J.A.,Rakowski, A. G. and Symons, R. H. (1994). A stable 463 nucleotide variant of citrus exocortis viroid produced by terminal repeats. J. Gen. Virol. 75, 727–732.
- Ding,S.-W.,Anderson,B.J.,Haase,H.R.and Symons, R. H. (1994). New overlapping gene encoded by cucumber mosaic virus genome. Virology 198, 593–601.
- Symons, R. H. (1994). Ribozymes. Current Opinion in Structural Biology 4, 322–330.
- Rakoswki, A. G. and Symons, R. H. (1994). Infectivity of linear monomeric transcripts of citrus exocortis viroid: Terminal sequence requirements for processing. Virology 203, 328–335.
- Rathjen, J. P., Karageorgos, L. E., Habili, N., Waterhouse, P. M. and Symons, R. H. (1994). Soybean dwarf luteovirus contains the third variant genome type in the luteovirus group. Virology 198, 671–679.
- Ding, S.-W., Rathjen, J. P., Li, W.-X., Swanson, R., Healy, H. and Symons, R. H. (1995). Efficient infection from cDNA clones of cucumber mosaic cucumovirus RNAs in a new plasmid vector. J. Gen. Virol. 76, 459–464.
- Ding, S.-W., Li, W.-X. and Symons, R. H. (1995). A novel naturally occurring hybrid gene encoded by a plant RNA virus facilitates long-distance virus movement. EMBO J. 23, 5762–5772.
- Ding, S.-W., Shi, B.-J., Li, W.-X. and Symons, R. H. (1996). An interspecies hybrid RNA virus is significantly more virulent than either parental virus. Proc. Nat. Acad. Sci. USA 93, 7470–7474.
- Warrilow, D. and Symons, R. H. (1996). Sequence analysis of the second largest subunit of tomato RNA polymerase II. Plant. Mol. Biol. 30, 337–342.
- Bonfiglioli, R. G., Webb, D. R. and Symons, R. H. (1996). Tissue and intracellular distribution of coconut cadang cadang viroid and citrus exocortis viroid determined by in situ hybridisation and confocal laser scanning and transmission electron microscopy. The Plant Journal 9, 457–465.
- Collins, N. C., Paltridge, N. G., Ford, C. M. and Symons, R. H. (1996). The Yd2 gene for barley yellow dwarf virus resistance maps close to the centromere on the long arm of barley chromosome 3. Theoret. Appl. Genetics 92, 858–864.
- Shi, B.-J., Ding, S.-W. and Symons, R. H. (1997). In vivo expression of an overlapping gene encoded by the cucumoviruses. J. Gen. Virol. 78, 237–241.
- Shi, B.-J., Ding, S.-W. and Symons, R. H. (1997). Two novel subgenomic RNAs derived from RNA 3 of tomato aspermy cucumovirus. J. Gen. Virol. 78, 505–510.
- Shi, B.-J., Ding, S.-W. and Symons, R. H. (1997). Plasmid vector for cloning infectious cDNAs from plant RNA viruses: high infectivity of cDNA clones of tomato aspermy cucumovirus. J. Gen. Virol. 78, 1181–1185.
- Ding, S.-W., Afsharifar, A., Shi, B.-J., Li, W.-X. and Symons, R. H. (1997). Recombinant viruses exhibiting a nonconventional type of virus synergy: The relevance to risk assessment of transgenic virus-resistant crops. In The Commercialisation of Transgenic Crops: Risk, Benefit and Trade Considerations, ed. G. D. McLean, P. M. Waterhouse, G. Evans and M. J. Gibbs, pp. 151–158 (Bureau of Resource Sciences, Canberra).
- Symons, R. H. (1997). Ribozymes to the fore. Nature 386, 141–142 (book review).
- Symons, R. H. (1997). Plant pathogenic RNAs and RNA catalysis. Nucleic Acids Res. 25, 2683–2689.
- Shams-Bakhsh, M. and Symons, R. H. (1997). Barley yellow dwarf virus-PAV RNA does not have a VPg. Arch. Virol. 142, 2529–2535.
- Ford, C. M., Paltridge, N. G., Rathjen, J. P., Moritz, R. L., Simpson, R. J. and Symons, R. H. (1997). Rapid and informative assays for Yd2, the barley yellow dwarf virus resistance gene, based on the nucleotide sequence of a closely linked gene. Molecular Breeding 4, 23–31.
- Liu, Y. and Symons, R. H. (1998). Specific RNA self-cleavage in coconut cadang cadang viroid: Potential for a role in rolling circle replication. RNA 4, 418–429.
- Lherminier, J., Bonfiglioli, R. G., Daire, X., Symons, R. H. and Boudon Padieu, E. (1998). Oligodeoxynucleotides as probes for in situ hybridization with transmission electron microscopy to specifically localize phytoplasma in plant cells. Mol. Cell. Probes 13, 41–47.
- Paltridge, N. C., Collins, N. C., Bendahmane, A. and Symons, R. H. (1998). Development of YLM, a codominant PCR marker closely linked to theYd2 gene for resistance to barley yellow dwarf disease. Theoret. Appl. Genet. 96, 1170–1177.
- Xin, H., Ji, L., Scott, S. W., Symons, R. H. and Ding, S. (1998). Ilar viruses encode a Cucumovirus-like 2b gene that is absent in other genera within the Bromoviridae. J. Virol. 72, 6956–6959.
- Wan Chow Wah, Y. F. and Symons, R. H. (1999). Transmission of viroids via grape seeds. J. Phytopathology 147, 285–291.
- Webb, D. R., Bonfiglioli, R. G., Carraro, L., Osler, R. and Symons R. H. (1999). Oligonucleotides as hybridization probes to localize phytoplasmas in host plants and insect vectors. Phytopathology 89, 894–901.
- Warrilow, D. and Symons R. H. (1999). Citrus exocortis viroid RNA is associated with the largest subunit of RNA polymerase II in tomato in vivo. Arch. Virol. 327, 1–9.
- Symons, R. H. (1999). Viroids. In Comprehensive Natural Products Chemistry, Vol. 6: Prebiotic Chemistry, Molecular Fossils, Nucleosides and RNA, pp. 169–187 (Elsevier).
- Symons, R. H. and Randles, J. W. (1999). Encapsidated circular viroid-like satellite RNAs. Current Topics in Microbiology and Immunology 239, 81–105.
- Symons, R. H. (1999). Ribozymes. In Encyclopedia of Virology, ed. A. Granoff and R. Webster, 2nd edition (Academic Press).
- Shi, B.-J., Palukaitis, P. and Symons, R. H. (2002). Differential virulence by strains of Cucumber mosaic virus is mediated by the 2b gene. Mol. Plant Microbe Interact. 15, 947–955.
- Shi, B.-J., Miller, J., Symons, R. H. and Palukaitis, P. (2003). The 2b protein of cucumoviruses has a role in promoting the cell-to-cell movement of pseudorecombinant viruses. Mol. Plant Microbe Interact. 16, 261–267.
- Shi, B.-J., Palukaitis, P. and Symons, R. H. (2004). Stable and unstable mutations in the 5/ non-translated regions of tomato aspermy virus RNAs 1 and 2 generated de novo from infectious cDNA clones containing a cauliflower mosaic virus 35S promoter. Virus Genes 28, 277–283.
- Shi, B.-J., Palukaitis, P. and Symons, R. H. (2005). The conserved, 5/ termini of RNA 1 and 2 of tomato aspermy virus are dispensable for infection but affect virulence. Virus Genes 30, 181–191.
- Shi, B.-J., Symons, R. H. and Palukaitis, P. (2007). The cucumovirus 2b gene drives selection of inter-viral recombinants affecting the crossover site, the acceptor RNA and the rate of selection. Nucleic Acids Research 35, 1–15.
Later years at the Waite Agricultural Research Institute, the wine industry and grape diseases
- Collins, G. G. and Symons, R. H. (1992). Extraction of nuclear DNA from grape vine leaves by a modified procedure. Plant Mol. Biol. Reporter 10, 233–235.
- Collins, G. G. and Symons, R. H. (1993). Polymorphisms in grapevine DNA detected by the RAPD PCR technique. Plant Mol. Biol. Reporter 11, 105–112.
- Bonfiglioli, R. G., Magarey, P. A. and Symons, R. H. (1996). PCR Analysis confirms an expanded symptomatology of Australian grapevine yellows. Aust. J. Grape and Wine Res. 1, 71–75.
- Bonfiglioli, R. G., Guerrini, S. and Symons, R. H. (1996). Cooperative Research Centre for Viticulture: Sampling program for Grapevine Yellows diseases. Australian Grapegrower and Winemaker 394, 22–24.
- Symons, R. H., Bonfiglioli, R. G., Habili, N. and Hamilton, R. P. (1996). It’s not worth the gamble: the penalties of using infected propagation material. Australian Grapegrower and Winemaker 396 (December), 13–15.
- Wan Chow Wah, Y. F. and Symons, R. H. (1997). A high sensitivity RT-PCR assay for the diagnosis of viroids in grapevines in the field and in tissue culture. J. Virol. Methods 63, 57–69.
- Bonfiglioli, R. G., Carey, C. T., Schliefert, L. F., Kinnear, A. J. and Symons, R. H. (1997). Description and progression of symptoms associated with grapevine yellows disease in young chardonnay vines in the Sunraysia region of Australia. Australian Grapegrower and Winemaker 400 (April), 11–15.
- Bonfiglioli, R. G., Habili, N., Schliefert, L. F. and Symons, R. H. (1997). Serious problems with top-working old vines: a warning to grapegrowers about the grapevine leafroll viruses. Australian Grapegrower and Winemaker 402 (June), 16–18.
- Habili, N., Bonfiglioli, R. G. and Symons R. H. (1997). Grapevine leafrollassociated virus 3 is in commercial vineyards – a cause for concern. Australian Grapegrower and Winemaker 405 (September), 39–40.
- Habili, N. and Symons, R. H. (1997). Leafroll virus major discussion topic at international virus seminar. Australian Grapegrower and Winemaker 409, 17–18.
- Habili, N., Bonfiglioli, R. G. and Symons, R. H. (1998). The trouble with Merlot. Australian Grapegrower and Winemaker 414a (June), 29–32.
- Habili, N., Bonfiglioli, R. G. and Symons, R. H. (1998). Rupestris stem pitting associated virus in Australia: Does it pose a threat to the viticultural industry? Australian Grapegrower and Winemaker 417 (September), 38–39.
- Bonfiglioli, R. G., HabiIi, N., Green, M., Schliefert, L. F. and Symons, R. H. (1998). The hidden problem: Rugose wood associated viruses in Australian viticulture. Australian Grapegrower and Winemaker 410 (December), 9–13.
- Symons, R. H. (1998). Waite Diagnostics: A service for the viticultural industry. Australian Grapegrower and Winemaker 417 (September), 44–46.
- Bonfiglioli, R. G., Habili, N., Rosa, C. and Symons, R. H. (1999) Viognier: Its viruses and its clonal identification. Australian Grapegrower and Winemaker 424 (April), 23–26.
- Habili, N. and Symons, R. H. (1999). Nested PCR, a highly sensitive technique for the detection of grapevine virus B. Australian Grapegrower and Winemaker 429 (September), 58–59.
- Constable, F. and Symons, R. H. (1999). Seasonal detection of phytoplasmas in Australian grapevines. Australian Grapegrower and Winemaker 429 (September), 49–53.






