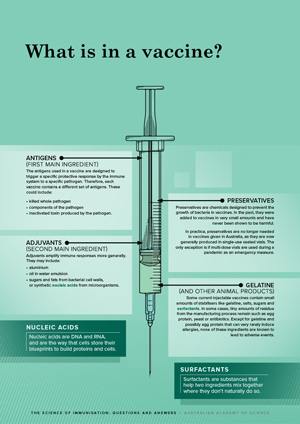
The antigens used in a vaccine are designed to trigger a specific protective response by the immune system to a specific pathogen.17 Therefore, each vaccine contains a different set of antigens.28–30 These could include:
Adjuvants amplify immune responses more generally. They may include:
Nucleic acids
Nucleic acids are DNA and RNA, and are the way that cells store their blueprints to build proteins and cells.
Preservatives are chemicals designed to prevent the growth of bacteria in vaccines. In the past, they were added to vaccines in very small amounts and have never been shown to be harmful.2,31
In practice, preservatives are no longer needed in vaccines given in Australia, as they are now generally produced in single-use sealed vials. The only exception is if multi-dose vials are used during a pandemic as an emergency measure.
Surfactants
Surfactants are substances that help two ingredients mix together where they don’t naturally do so.
Some current injectable vaccines contain small amounts of stabilisers like gelatine, salts, sugars and surfactants.32,33 In some cases, tiny amounts of residue from the manufacturing process remain such as egg protein, yeast or antibiotics. Except for gelatine and possibly egg protein that can very rarely induce allergies, none of these ingredients are known to lead to adverse events.
Some vaccines contain the killed whole microorganism that the vaccine is designed to protect against. The virus or bacterium is grown in a laboratory and killed by heat or chemicals so that it is no longer infectious.34 The injectable polio vaccine and inactivated hepatitis A vaccine are examples of this type.
Other vaccines contain only components of the pathogen as their antigens. These components can be prepared by purifying them from the whole bacterium or virus, or by genetically engineering them.35–37 Engineered vaccines include the human papillomavirus vaccine, which protects against cervical cancer, and the hepatitis B virus vaccine.
In some vaccines, components of the pathogen are linked with proteins to create an antigen that can generate a stronger response—this allows even 6-week-old babies to make significant amounts of antibodies, which they otherwise could not do until they are older.38 These vaccines are called conjugate vaccines and include those against Haemophilus influenzae type b (Hib) infection, meningococcal and pneumococcal disease.
Another group of vaccines is based on the toxin produced by the pathogen that causes the disease symptoms. The toxin is chemically treated to make it harmless. The antibodies produced against it can still neutralise the toxin and prevent disease symptoms from developing. Examples of this type include tetanus and diphtheria vaccines.
Attenuation
The attenuation process permanently alters the pathogen so that it is still able to reproduce and stimulate an immune response, but does not cause disease.
Some vaccines contain an infectious microorganism; these are called live vaccines. The microorganism may be derived from the pathogen that the vaccine aims to protect against. This is usually achieved by growing the pathogen in the laboratory under conditions designed to weaken or attenuate it.39 Examples include the injectable MMR vaccine and the chickenpox vaccine.
Alternatively, a live vaccine may consist of a naturally occurring organism closely related to the pathogen that does not cause disease in healthy humans with intact immune systems. An example is the BCG vaccine against tuberculosis and leprosy.
Vaccines containing live pathogens are not recommended for people whose immune systems are impaired due to the use of immunosuppressive drugs, serious illness or abnormalities of the immune system, because of the risk of causing disease. Similarly, live vaccines are not recommended during pregnancy as a precautionary measure, in case the pathogens they contain cross the placenta to the unborn baby. This is because a baby’s immune system is not completely developed until after birth. Vaccines without live microorganisms (inactivated vaccines), in contrast, have not been shown to be harmful in pregnancy.
Adjuvants are not needed in vaccines that use live organisms, as these naturally produce inflammation and amplify protective immunity.
Adjuvants are substances that promote a more vigorous immune response to vaccine antigens. They can also help target the body’s response. In doing so, they may cause mild local reactions (soreness, redness and swelling) at the injection site. These reactions are expected and part of the innate immune response.
Most inactivated vaccines use adjuvants to make the body’s defences think a significant infection is present. They stimulate stronger, longer-lasting immune responses to the vaccine antigens, leading to better protection against subsequent infection. Adjuvants are not needed in vaccines that use live organisms, as these naturally produce inflammation and amplify protective immunity.
Aluminium salt (known as alum) has been frequently used in human vaccines as an adjuvant and has a track record of safety dating back to the 1950s.40 Some newer vaccines incorporate more active adjuvants, derived from naturally-occurring oil in water emulsions (mixtures), fats or sugars from bacterial cell walls, or synthetic nucleic acids mimicking bacterial DNA. These can produce more vigorous and better-targeted immune responses against the infectious agent.41
In addition to adjuvants and antigens, vaccines can contain tiny quantities of materials from the manufacturing process. These can include trace amounts of detergents, nutrients from the laboratory cultures, chemicals used to kill the pathogens, stabilisers like gelatine, or small amounts of DNA and parts of dead organisms.
Vaccine developers are required by regulatory authorities to test for the presence of these extra materials during the manufacturing process to ensure they do not exceed levels known to be safe.
Occasionally, individuals can be allergic to a vaccine ingredient, although such reactions are rare. Reviews of vaccine monitoring data have shown that less than 1 out of 100,000 people might experience a severe allergic reaction.42,43 Vaccines do not cause allergic diseases.
The presence of DNA or RNA (genetic instructions) in a vaccine does not lead to changes to the DNA or RNA of the person who receives it.
Because most vaccine antigens are prepared from whole organisms, a vaccine may contain some of that organism’s genetic material in the form of DNA, or a similar type of molecule known as RNA. The amount of genetic material in a vaccine is minuscule, much less than the amount we eat in our food each day.44 Vaccines based on living pathogens contain that organism’s genetic information because it is necessary for the vaccine to work. However, the DNA (or RNA) in the pathogen does not remain for long or lead to long-term detrimental effects in the vaccinated person.45 The presence of DNA or RNA in a vaccine does not lead to changes in the vaccinated person’s DNA or RNA.
Some vaccines, such as influenza or MMR vaccines, contain antigens from viruses grown in eggs or on chick cells and may contain some egg proteins. However, newer MMR vaccines contain so little egg protein that it is now considered safe to give them even to someone who is already known to be very sensitive to egg protein.46 The seasonal influenza vaccines in current use contain minimal amounts of egg protein and can be used in most egg‑sensitive people.47
The viruses in two other less-frequently-used vaccines (for Q fever and yellow fever) are also grown in eggs, and specialist advice should be sought if either of these vaccines are needed for a person with severe egg allergy.
Small amounts of gelatine of animal origin are used to stabilise some vaccines like Zostavax and MMR. Allergic reactions to gelatine have been reported, but are rare and usually mild in recipients of these two vaccines. However, in people with mammalian meat allergy, the chance of serious anaphylactic reactions is increased. Specialist advice should be sought in such cases before administering a vaccine like Zostavax, the chickenpox and shingles vaccine.
Fetus
Fetus is a term used to describe a baby before they are born.
Certain viruses grown for use in vaccines require the use of ‘cell lines’. These cell lines were derived over 40 years ago from human fetal tissue. Vaccines manufactured using these cell lines include those for rubella, chickenpox, shingles and hepatitis A. Copies of these cells are still used because they are excellent viral factories and are free of contaminants, which can be a problem when viruses are grown in cells that are not from humans. Once the cell has been used to grow the virus, the virus is taken out and purified—the human cells are not included in the vaccine. It is estimated that the vaccines made using cells from these original fetal tissue samples have prevented nearly 11 million deaths and prevented at least 4 billion cases of disease.
Vaccines are commonly given by injection into a muscle—this is called intra-muscular injection. Some vaccines can be injected into the layer of fat just under the skin—this is called subcutaneous injection. Some vaccines can be given orally (through the mouth) instead of as an injection, for example the oral polio vaccine.
Vaccination sets off a chain of events resulting in movement of immune system cells to the area where the vaccine was administered, then through to the lymph nodes and spleen, eventually leading to the development of immunity throughout the body. Vaccines are not delivered directly into the bloodstream. One common side effect of intramuscular injection is some short-term discomfort at the injection site. Severe side effects, however, are very rare.

© 2025 Australian Academy of Science